Is Sif4 Polar Or Nonpolar
Is SiF4 polar or non-polar. Get the detailed answer.

Ccl4 Lewis Structure Carbon Tetachloride In 2021 Carbon Molecule Molecules Lewis
If you want to quickly find the word you want to search use Ctrl F then type the word you want to search.

Is sif4 polar or nonpolar. SiF4 is a nonpolar molecule because the fluorines are arranged around the central silicon atom in a tetrahedral molecule with all of the regions of negative charge cancelling each other out. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. SiF4 is a non-polar molecule.
Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. SF4 Sulfur tetrafluoride is polar in nature as sulfur atom consists of a lone pair on it due to which the shape of the molecule becomes asymmetric ie. Their bond dipoles do not cancel so the molecule is polar.
Though Si-F bonds are polar but the molecule exhibit tetrahedral structure cancelling the dipole moment. Its boiling point is very low around 4 C. SiF4 Dot Lewis Structure Molecular Geometry Bond Angle Polar or Nonpolar.
But the other two S-F dipoles are pointing down. In VSEPR theory the lone pair forces the molecular geometry of SF4 into a see-saw shape. Hello EveryoneIn todays video we are going to help you determine the polarity of the SiF4 molecule.
It is a colorless and volatile Compound. Silicon Tetrafluoride In todays video we are going to help you determine the polarity of the SiF4 molecule. SiF4 is not polar as the fluorines negative dipoles cancel each other out as the are all pulling away form the centre equally the centre being silicon which has a lower electronegativity than fluorine.
Is SiF 4 polar or non. It causes charges produced on the molecule to cancel each others out and hence net becomes zero. It is a chemical formula for Silicon Tetrafluoride.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Some Useful Properties of SiF4. This is because SiF4 has tetrahedral molecular geometry which means the molecule is symmetrical so that the polarities of the Si-F bonds cancel each other and the net dipole becomes zero.
Also Know does silicon tetrafluoride have a dipole moment. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Answer SeF4 is Polar What is polar and non-polar.
Answer SOF4 Thionyl tetrafluoride is Polar What is polar and non-polar. Is SiF4 polar or non-polar. For SiF4 are the bonds polar or nonpolar.
Then we will study the polarity of SiF4 ie whether SiF4 is a polar or nonpolar molecule. The molecule SiF4 has four bond pairs and zero lone pairs and hence being symmetrical it is non polar and has zero dipole moment. Moreover Fluorine is more electronegative than Sulfur due to which the overall charge distribution of a molecule is uneven resulting in a polar molecule and give 0632 D dipole moment.
SiF4 Silicon tetrafluoride is Nonpolar. Yes SiF4 is a nonpolar molecule despite four individual Si-F bonds are polar in nature. The geometrical symmetry of Sif4 plays vital role in making it a non-polar molecule.
By signing up youll get thousands of step-by-step solutions to your homework. Free unlimited access for 30 days limited time only. As mentioned SiF4 have tetrahedral shape And in Non-polar.
Get the detailed answer. Log in Sign up. So net dipole is 0.
SiF4 Lewis Structure First of all there is a need to understand the Lewis structure of silicon tetrafluoride for studying its chemical bonding. Question Is SeF4 polar or nonpolar. In case of SiF4 the central atom Silicon belongs to Carbon family and has 4 Valence electrons.
List molecules polar and non polar. So there is no dipole moment observed. So is SF4 Polar or Nonpolar.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Is SiF4 Polar or Non-polar. Ill tell you the polar or nonpolar list below.
Two of the S-F bonds are pointing away from each other and their bond dipoles cancel.

Ccl4 Lewis Structure Carbon Tetachloride In 2021 Carbon Molecule Molecules Lewis

Of The Molecules Sif4 And Sibr4 Which Has Clutch Prep

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

Science Coverage Is Clo3 Polar Or Nonpolar In 2021 Molecular Geometry Covalent Bonding Oxidation State

Is Chf3 Polar Or Nonpolar Fluoroform Youtube

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules

Science Coverage Is Hf Polar Or Nonpolar Electron Affinity Covalent Bonding Molecular Geometry

Molecule Or Polyatomic Ion Polar Or Nonpolar Atom Chegg Com

Science Coverage Is Cf2cl2 Polar Or Nonpolar Molecular Geometry Covalent Bonding Solubility

Polar Or Non Polar That Is The Question Ppt Download

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

Science Coverage Is Asf5 Polar Or Nonpolar In 2021 Molecular Geometry Molar Mass Octet Rule
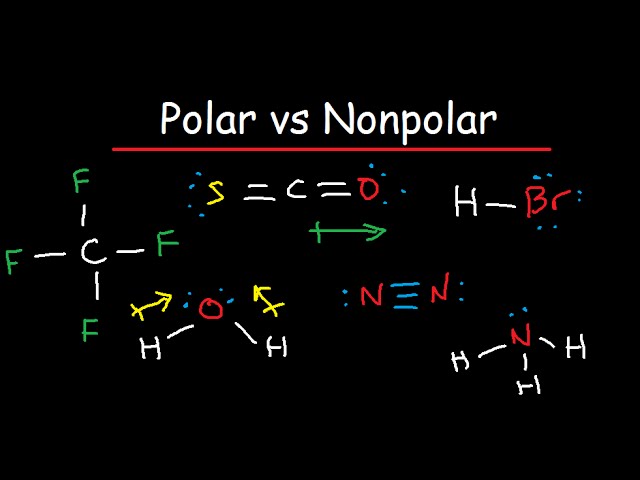
Polar And Nonpolar Molecules Is It Polar Or Nonpolar Youtube

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots
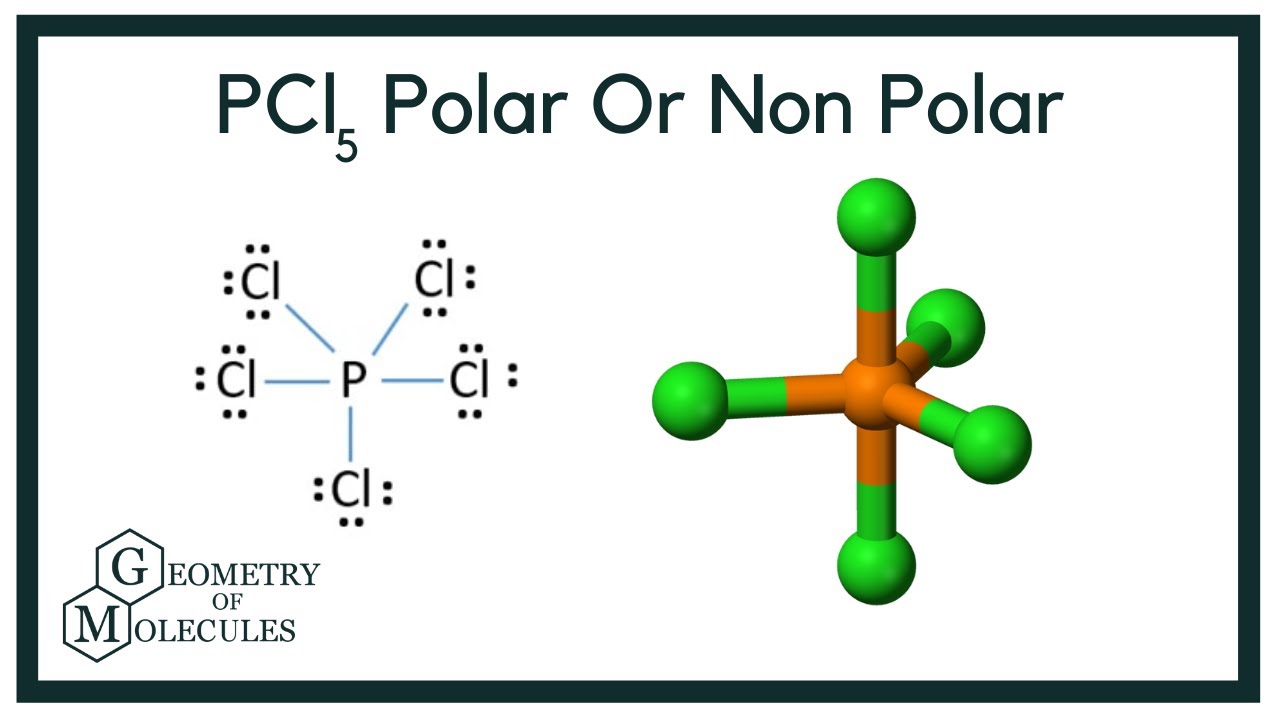
Is Pcl5 Polar Or Nonpolar Phosphorous Pentachloride Youtube

Nacl Polar Or Nonpolar Sodium Chloride In 2021 Math Equations Molecules Sodium

