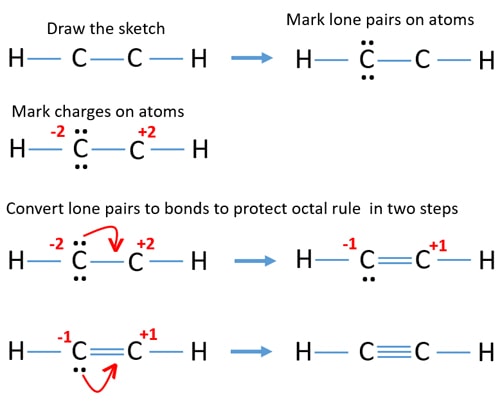
Lone Pairs In C2h2
Since C2H2 is a linear molecule the C must be sp. You do not have to include lone pairs in your answer.

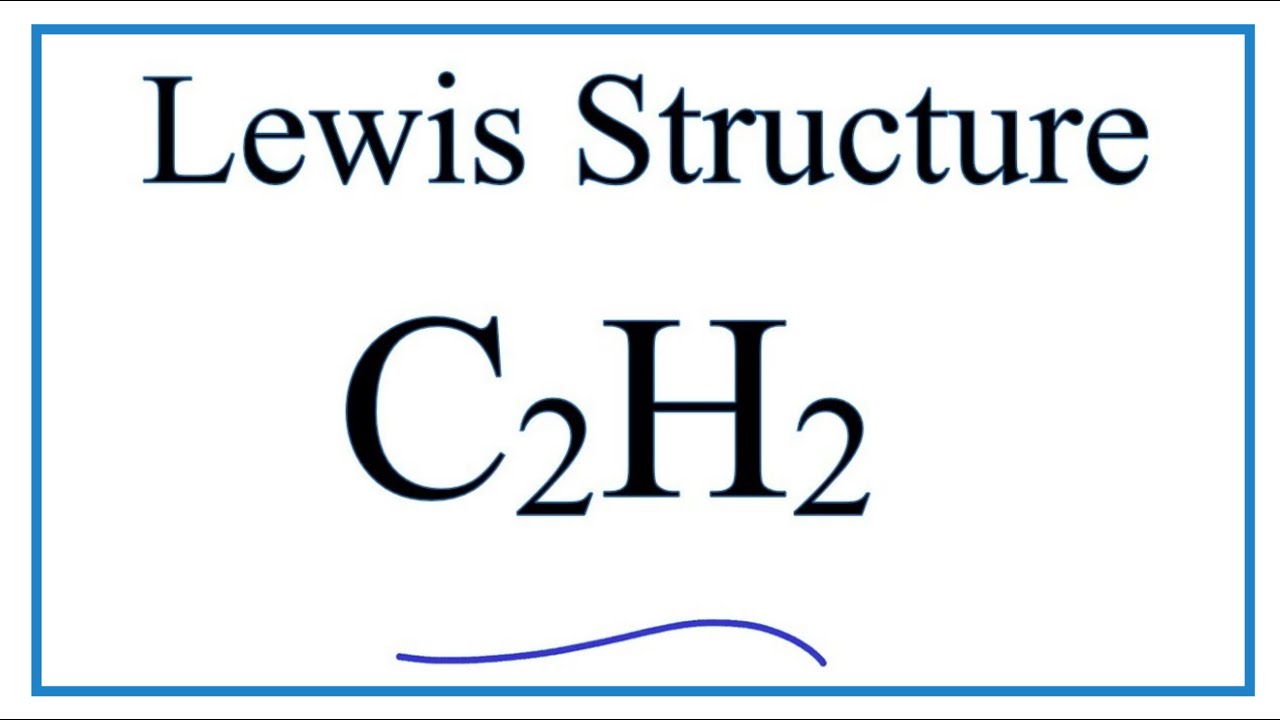
C2h2 Acetylene Ethyne Lewis Structure
Other examples include CO2 HCN C2H4 C2H2.

Lone pairs in c2h2. Is C2H2 a triple bond. For NH3 it has 3 covalent bonds and a lone pair so the molecule will look as a trigonal pyramid For BH3 it is the central atom and has no lone pair apart from the three bonds so the molecule will look as a trigonal planar. The first oxygen has three bonds the second only has one.
What is C2H2 used for. There are no lone pairs in the central atom of boron trichloride because it is one of the exceptions in the octet rule. Explicitly draw all H atoms.
CO2 has two regions of electron density and no lone pairs on the central C atom so by VSEPR Theoey the molecule is linear. How Many Valence Electrons Are In Lone Pairs In The Lewis Dot Structure Of C2H2. Arrange electrons until both carbon and nitrogen get a triple bond giving an octet and hydrogen has 2.
4 bonding pairs and 1 lone pair. C 4A 2 4 e 8 e H 1A 2 1. Ethyne is also called Acetylene.
Quarterfreelp and 20 more users found this answer helpful. You can think of the reaction taking place by a lone pair on the oxygen of one water molecule ripping off the proton only of the hydrogen of another water molecule to form a covalent bond between them using just the lone pair. In this tutorial we are going to learn how to draw the lewis structure of C 2 H 2 step by step.
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. C2H2 is a linear molecule as the distribution of the atoms is symmetric in this molecule. How many lone pairs are in bcl3.
What is the best description of. Used in oxy-acetylene torches used for welding. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds.
C2H2 Molecular Geometry And Bond Angles As a result of the double bond C2H2 molecular geometry is linear with a bond angle of 180o. Its chemical formula is C2H2. The Carbon Atoms Are Bonded To Each Other And Each Hydrogen Atom Is Bonded To A Carbon Atom.
The O in HOCl has two lone pairs and two bonding pairs in a tetrahedral arrangement which is sp3. How many types of law are there in physics. There are no lone pairs on carbon or hydrogen atoms.
Is C2H2 a linear molecule. Draw a line-bond structure for C2H2. The Carbon Atoms Are Bonded To Each Other And Each Hydrogen Atom Is Bonded To A Carbon Atom.
There are no lone pairs on carbon or hydrogen atoms. C2H2 a How many lone pairs non-bounding electron pairs does the compound possess on All atoms. How many bonding pairs and lone pairs of electrons surround the sulfur atom in the SF4 molecule.
The O in HOCl has two lone pairs and two bonding pairs in a tetrahedral arrangement which is sp3. Hydrogen has one bond and no lone pairs. In cases where there is more than one.
It is a colourless inflammable gas widely used as a fuel in oxyacetylene welding and cutting of metals and as raw material in the synthesis of many organic chemicals and plastics. Sp2 carbon would give a trigonal planar arrangement. These electrons are known as the valence electrons.
Lewis Structures - Dot Diagram Formal Charge Molecular. B For this compound Identify the following -number of electron groups electron domains -number of atoms bounded to the central atom -number of non-bounding electron pairs lone pairs attached to the central atom -General formula c What is the Electron group. H2O has four regions of electron density and two lone pairs on the central O atom so the molecule is bent.
Put carbon in the center and arrange hydrogen and nitrogen atoms on the sides. Draw the best Lewis structure for C2H2 by filling. Chemical Nameformula Valence Electrons Hybridi.
Also only sp carbon can form a triple bond. There are 2 bond pairs between carbon atoms and 4 bond pairs between carbon and hydrogen atomsNo lone pair. A quick explanation of the molecular geometry of C2H2 including a description of the C2H2 bond angles.
Which molecule has the shortest bond between carbon atoms. Include all lone pairs of electrons. Central atoms and outer atoms.
C2h2 Acetylene Ethyne Lewis Structure
Lewis Structure For C2h2 Ethyne

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
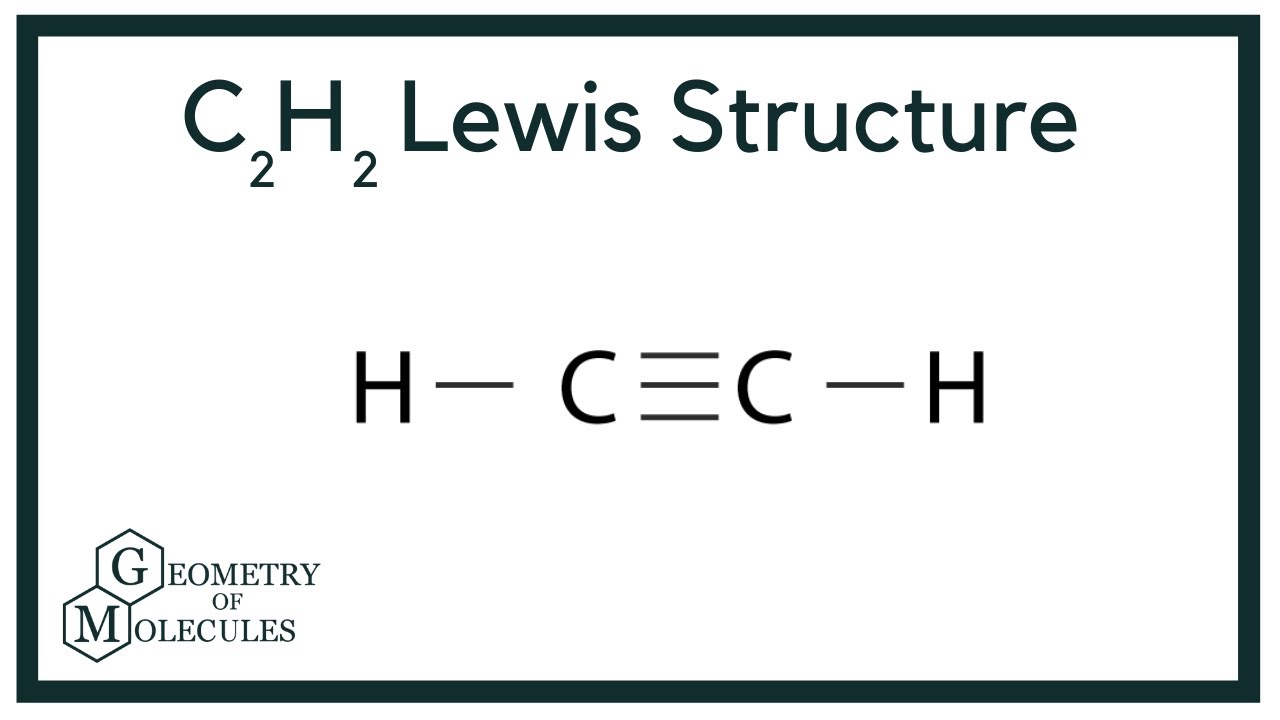
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
C2h2 Polar Or Nonpolar Check Dipole Moment And Polarity Geometry Of Molecules

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

Below Is The Lewis Structure Of The Acetylene Chegg Com
How To Detect The Hybridization Of Acetylene Quora
Chem 210 Chapter 1 Review Quiz

Below Is The Lewis Structure Of The Acetylene C2h2 Chegg Com

Molecular Orbital Theory Ppt Download

Molecular Geometry Of Acetylene Chemistry Stack Exchange

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com

Answered C2h2 A How Many Lone Pairs Bartleby

C2h2 Acetylene Ethyne Lewis Structure

C2h2 Molecular Geometry Shape And Bond Angles See Description For Note Youtube
C2h2 Molecular Geometry Shape And Bond Angles See Description For Note دیدئو Dideo
