C2cl2 Lewis Structure
During the next 96 hr elimination of retained approximately 17 20 and 40 ppm doses respectively was. Draw the Lewis structure for the molecule.
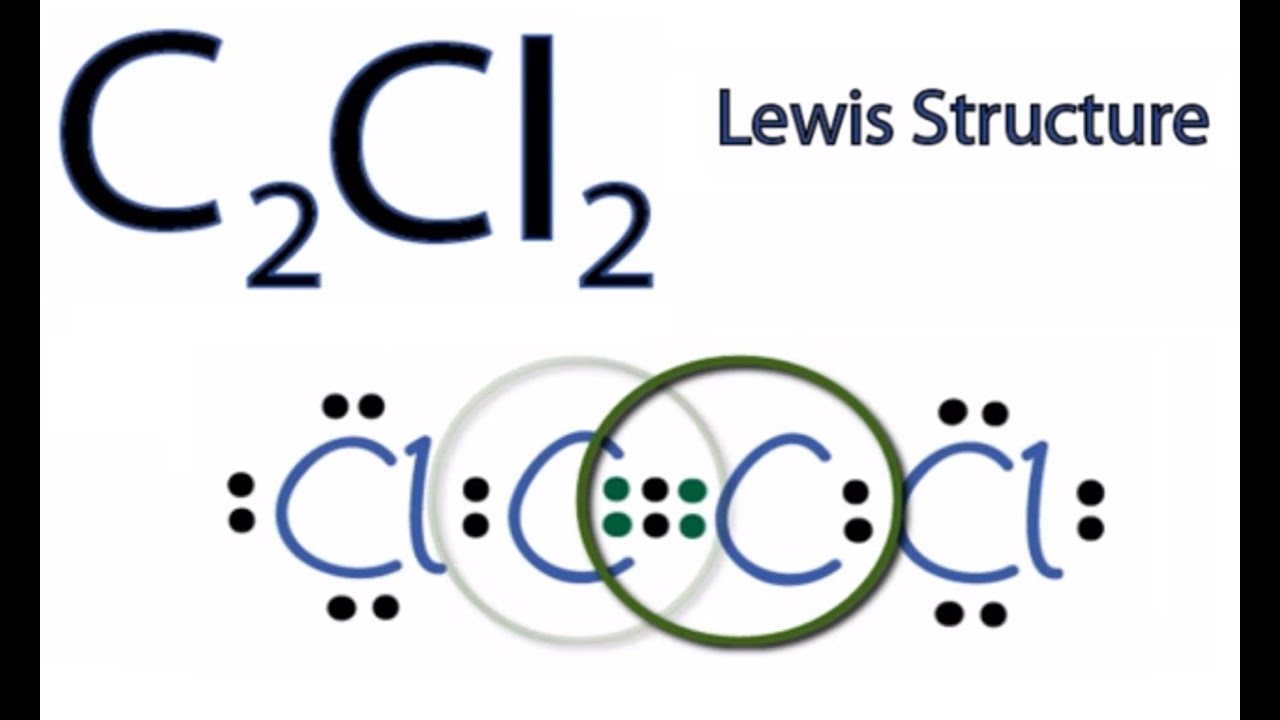
C2cl2 Lewis Structure How To Draw The Lewis Structure For C2cl2 Youtube
For the following species.

C2cl2 lewis structure. Draw a 3D perspective drawing using the wedge and dash format. Hydrogen is usually surrounded by 4 electrons in a valid Lewis structure. If you are struggling to do this look at the structural formula and replace the bonds and lone pairs with dots.
Draw the Lewis structure for the species including all isomers andor resonance forms. Its primary use has been as a refrigerant. CF2Cl2 is made up of three distinct atoms C Cl and F.
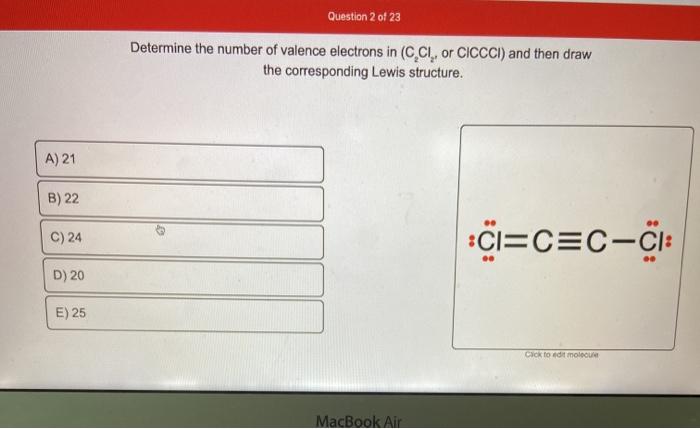
The molecular shape of the C2Cl2 molecule is linear. O2 BBr3 H3O NOF IF2 AsO43 HCO3 HBrO3 CH2F2 CH2O2 C2Cl2 C2H3F3 C2H2F2 1. Subtract one electron for each positive charge.
That would be the same as any dot structure. Put our Hydrogens here and then our Chlorines. Total number of Valence electrons 4 21 27.
The metabolism of inhaled 14 Cdichloroacetylene has been studied in male Wistar rats exposed to 20 or 40 ppm 78 or 156 mgcu m atmospheres for 1 hr. Write the correct skeletal structure. Determine the central atom in this molecule.
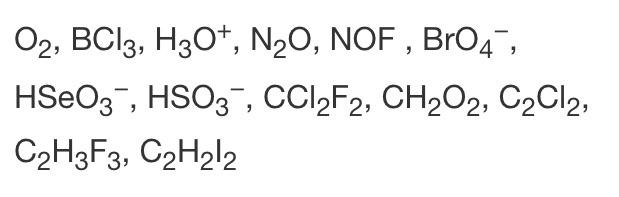
For mathN_2Cl_2math you can look at dinitrogen difluoride as providing your structur. Determine the total number of valence electrons from all of the atoms in the molecule or ion. Lewis Structure Review How To Write Lewis Structures 1.
Draw a Lewis structure for CF2Cl2 determine its geometry and determine whether the molecule is polar. A single bond in a Lewis structure represents 2 electrons. And a melting point of -967 C.
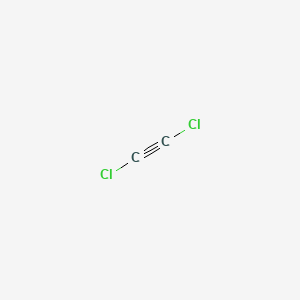
Draw the Lewis structure of C 2 CL 2 and then determine if the molecule is polar or nonpolar. Starting with its Lewis structure the C2Cl2 molecule has a total of 22 valence electrons 4 from each of the two carbon atoms and 7 from each of the two chlorine atoms. Alternatively a dot method can be used to draw the lewis structure of COCl 2.
BrCl3 Discuss briefly the relationship between the dipole moment of a molecule and the polar character of the bonds within it. It is a non-flammable gas with a sweetish chloroform -like odor with the critical point occurring at 1456 C and 326 MPa. Answer CH2Cl2 DICHLOROMETHANE is Polar What is polar and non-polar.
To do so we first need to do the following steps. Lewis dot structure of CO Cl 2. Become a member and unlock.
It is widely used as a solvent in chemistry laboratories. Add one electron for each unit of negative charge. This molecule has a total of 26 valence electrons six.
Select all the correct answers. Carbon has four valence electrons Hydrogen has one valence electrons and like all halogens Chlorine has seven valence electrons. Tetrachloroethylene-13C1 C2Cl4 CID 12233487 - structure chemical names physical and chemical properties classification patents literature biological.
12-Dichlorotetrafluoroethane or R-114 also known as cryofluorane INN is a chlorofluorocarbon CFC with the molecular formula ClFCCFCl. For molecules of the formula ABn place the least electronegative element in the center. Identify the true statement about Lewis structures.
Dichloromethane or methylene chloride with the chemical formula CH2Cl2 is a colorless volatile liquid with a boiling point of 396 C. CH2Cl2 Lewis Structure Molecular Geometry Hybridization and MO Diagram. When we talk about CH2Cl2 Carbon is less electronegative than Chlorine atoms.
A step-by-step explanation of how to draw the CH2Cl2 Lewis Dot Structure DichloromethaneFor the CH2Cl2 structure use the periodic table to find the total. Zero and it behaves as a non polar molecule. See full answer below.
We have a total of 20 valence electrons for CH2Cl2. C4O6Cl2x714 Total24 Put carbon in the center. To understand the Lewis structure lets first calculate the total number of valence electrons for Dichloromethane.
Calculate the total number of valence electrons present. This is the Lewis structure for CH2Cl2. Disulfur dichloride S2Cl2 S 2 C l 2 has two central S atoms each bonded to a Cl atom.
For the CF 2 Cl 2 Lewis structure there are a total of 32 valence electrons available. Were being asked to draw the 3 Lewis structures for C 2 H 2 Cl 2 and indicate whether each is non-polar or polar. Calculate the total valence electrons in COCl 2 molecule.
Carbon is less electronegative than Chlorine so itll go on the inside and Hydrogens always go on the outside. It is polar because of the presence of two chloro groups but is not miscible with water.
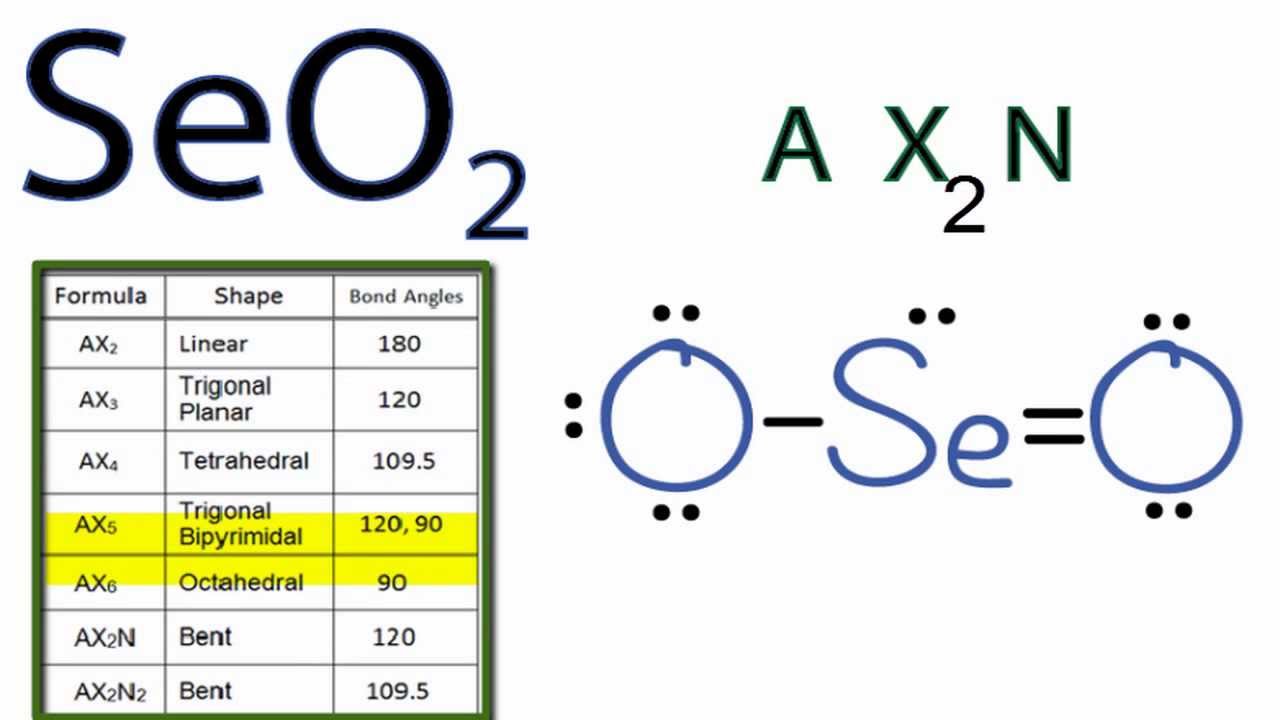
Seo2 Molecular Geometry Shape And Bond Angles Youtube
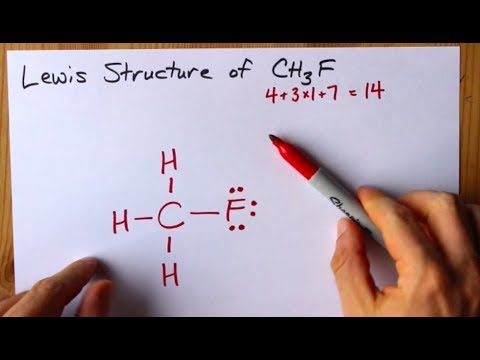
How To Draw The Lewis Structure Of Ch3f Fluoromethane Youtube
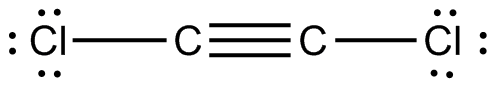
What Is The Molecular Shape Of C2cl2 Socratic

Video Lewis Dot Structure For Pbr3

Chapter 8 Chemical Bonding Study Guide Flashcards Quizlet
Draw The Lewis Structure For The Following Include Chegg Com

C2cl4 Lewis Structure How To Draw The Lewis Structure For Tetrachloroethylene Youtube
Cl3po Lewis Structure How To Draw The Lewis Structure For Cl3po Youtube

C2cl4 Lewis Structure How To Draw The Lewis Structure For Tetrachloroethylene Youtube
How To Draw The Lewis Dot Structure For Seo3 2 Selenite Ion Youtube
Dichloroacetylene C2cl2 Pubchem

Lewis Vsepr And Contour Diagrams For C2cl2 Youtube

Determine The Hybridization Of The Following Molecules A C2cl2 B Co2 C O3 D H2o Study Com
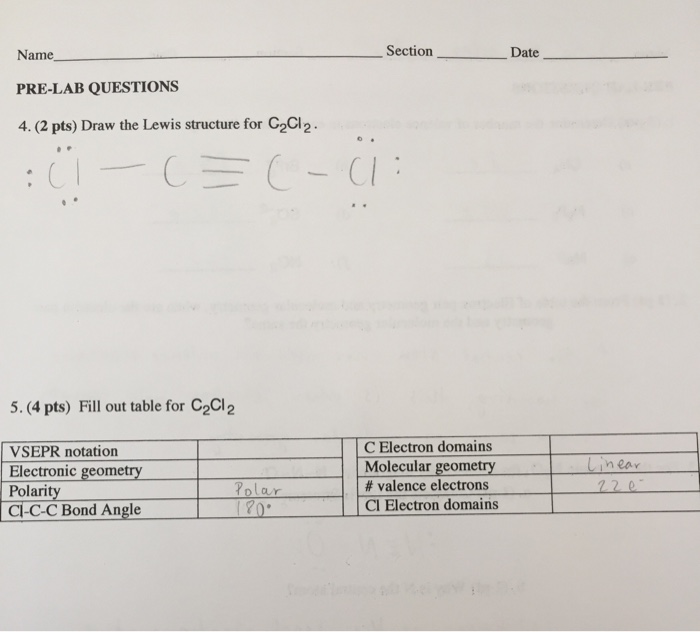
Section Date Name Pre Lab Questions 4 2 Pts Draw Chegg Com
Question 2 Of 23 Determine The Number Of Valence Chegg Com
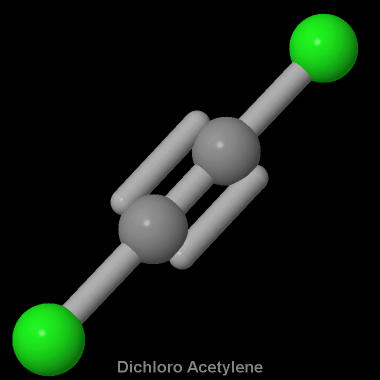
What Is The Molecular Shape Of C2cl2 Socratic