Is Pf5 A Lewis Base
Why is PCl3 a Lewis base. Im Gegensatz zur Definition nach Brønsted bei der als Maß für die Stärke einer Säure oder Base ihre Säurekonstante bzw.

Phosphorpentafluorid Chemie Schule
For example PF5 F- Pf6 is a lewis acid-base reaction.

Is pf5 a lewis base. Compounds of the chalcogens oxygen sulphur selenium and tellurium in which they exhibit an oxidation state of -2 generally act as Lewis bases. An atom ion or molecule with a lone-pair of electrons can thus be a Lewis base. None of the above is a Lewis acid.
The phosphorus in PCl5 readily accepts electrons from other molecules. Draw molecular geometries for the reactants and the product ofthe reaction of PF5 with the Lewis base NH3. Phosphorus trichloride has a lone pair and therefore can act as a Lewis base.
My answer is option 2 but the answer provided is 4 where am I going wrong. Behaves as a Lewis Acid showing electron-accepting properties generally in a ratio of 11 with Lewis bases. Typische Lewis-Basen sind zB.
It forms complexes with amines ethers nitriles sulfoxides and other bases. Phosphorpentafluorid Phosphorpentafluorid Phosphor V-fluorid ist eine anorganische chemische Verbindung aus den Elementen Phosphor und Fluor mit der Summenformel PF 5 und zählt zur Verbindungsklasse der Phosphorhalogenide. Is pcl5 a Lewis base.
Each of the following anions can give up their electrons to an acid eg OH CN CH3COO NH3 H2O CO. They utilize the highest occupied molecular orbital or HOMO Figure 2. A Lewis base is a chemical compound that can donate a pair of electrons to a suitable electron-pair acceptor Lewis acid to form a Lewis adduct.
I believe that thats correct where Arrhenius and Bronsted is a reaction with water but I believe that lewis acids and bases dont necessarily have to be in water. Therefore it is considered as a Lewis acid. NH 3 H 2 O F - CN - oder CO.
A Lewis base is a chemical compound that can donate a pair of electrons to a suitable electron-pair acceptor Lewis acid to form a Lewis. Die Paarung zweier harter. It acts as a mild Lewis acid.
Fastest Reliable Cheapest Game Keys and Digital Services at DamnModz. 3 question Phosphorus pentafluoride PF5 acts as a during the formation of the anion PF6. Ihre Basenkonstante dient muss man für Lewis-Säuren und Lewis-Basen neben einer quantitativen Einordnung nach stark und schwach zusätzlich eine qualitative Klassifizierung nach hart und weich treffen HSAB-Konzept auch Pearson-Konzept genannt.
Its also a pi acceptor Lewis acid. S b C l X 3 has a completely filled spd orbitals so it can no longer accept electrons hence doesnt act as a Lewis acid. Yes ethyl acetate or ethyl ethanoate is a Lewis base since it has the ability to act as an electron-pair donor.
Find more Chemistry widgets in. Select the correct answer below. Lewis Bases are Nucleophilic meaning that they attack a positive charge with their lone pair.
Is ethyl acetate a Lewis base. The fluorides BF3 AIF3 SIF4 and PF5 are Lewis acids. Lewis Bases donate an electron pair.
Include geometry andformal charges in. For example with the Lewis. Es ist bei Standardbedingungen ein farbloses sehr giftiges und nicht brennbares Gas mit stechendem Geruch.
Im Gegensatz zur Lewis-Säure ist eine Lewis-Base ein Elektronenpaardonator. According to the Lewis concept acid is the substance that accepts a lone pair of electrons since it has empty orbitals in the valence shell. Lewis-Basen sind Atome oder Moleküle die ein freies Elektronenpaar besitzen das zur Ausbildung einer kovalenten Bindung Atombindung geeignet ist.
Chemistry learning made easyThis tutorial will help you deal with the lewis structure and moleculargeometry for phosphorus pentafluoride PF5.
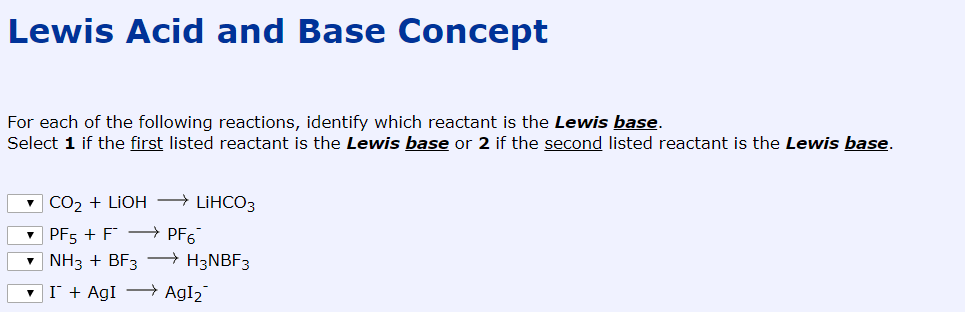
Lewis Acid And Base Concept For Each Of The Following Chegg Com
Is It True That Pf5 Is A Polar Molecule Quora

1 8 Chemistry 2810 Answers To Assignment 3 Topic Lewis
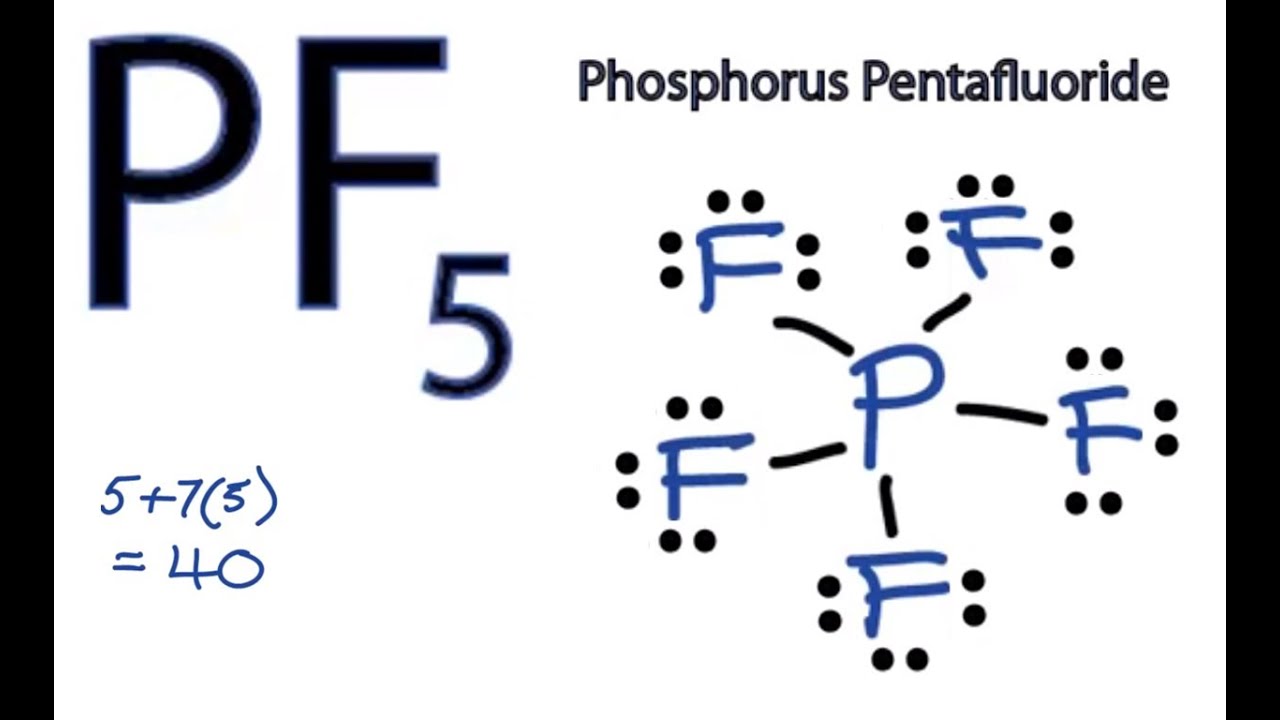
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
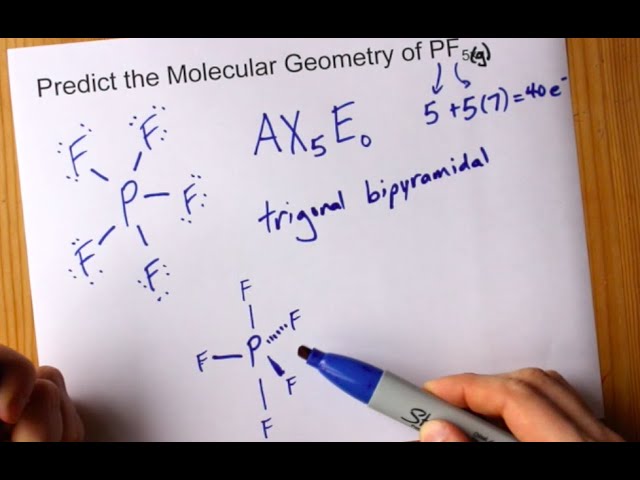
Molecular Geometry Of Pf5 Phosphorus Pentafluoride Youtube
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora
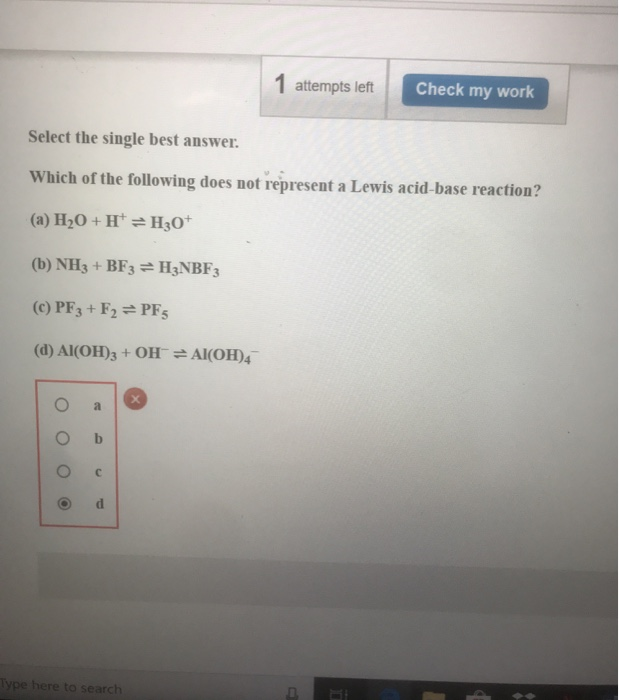
1 Attempts Left Check My Work Select The Single Best Chegg Com

Pf5 Lewis Structure And Molecular Geometry Made Easy Youtube
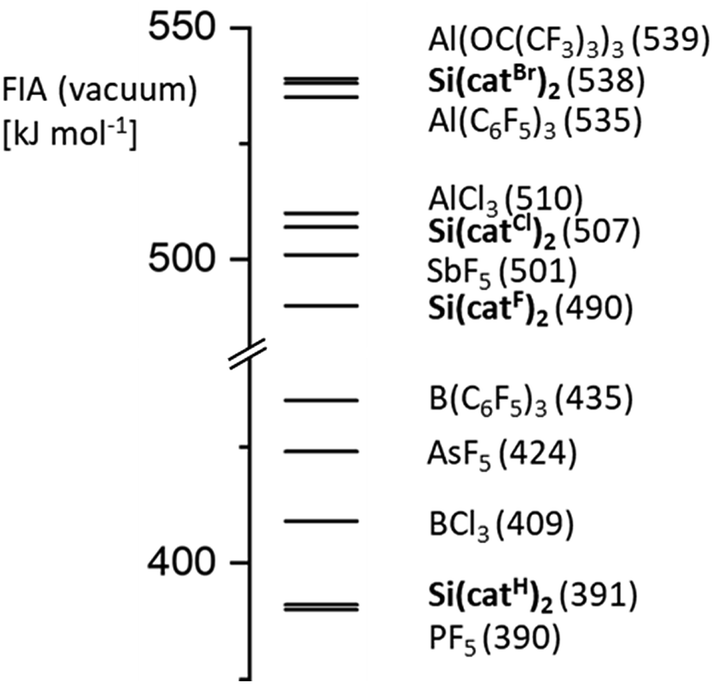
Bis Catecholato Silanes Assessing Rationalizing And Increasing Silicon S Lewis Superacidity Chemical Science Rsc Publishing Doi 10 1039 C9sc02167a

Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
Http Www Carbene De Wp Content Cm 1111 Tutorial 6 With Answers Pdf
Solved The Uorides Bf3 Aif3 Sif4 And Pf5 Are Lewis Acids The All Form Very Stable Uoroanions When Treated With Lithium Uoride In Contrast The Course Hero

Pf5 Lewis Structure Phosphorus Pentafluoride Youtube
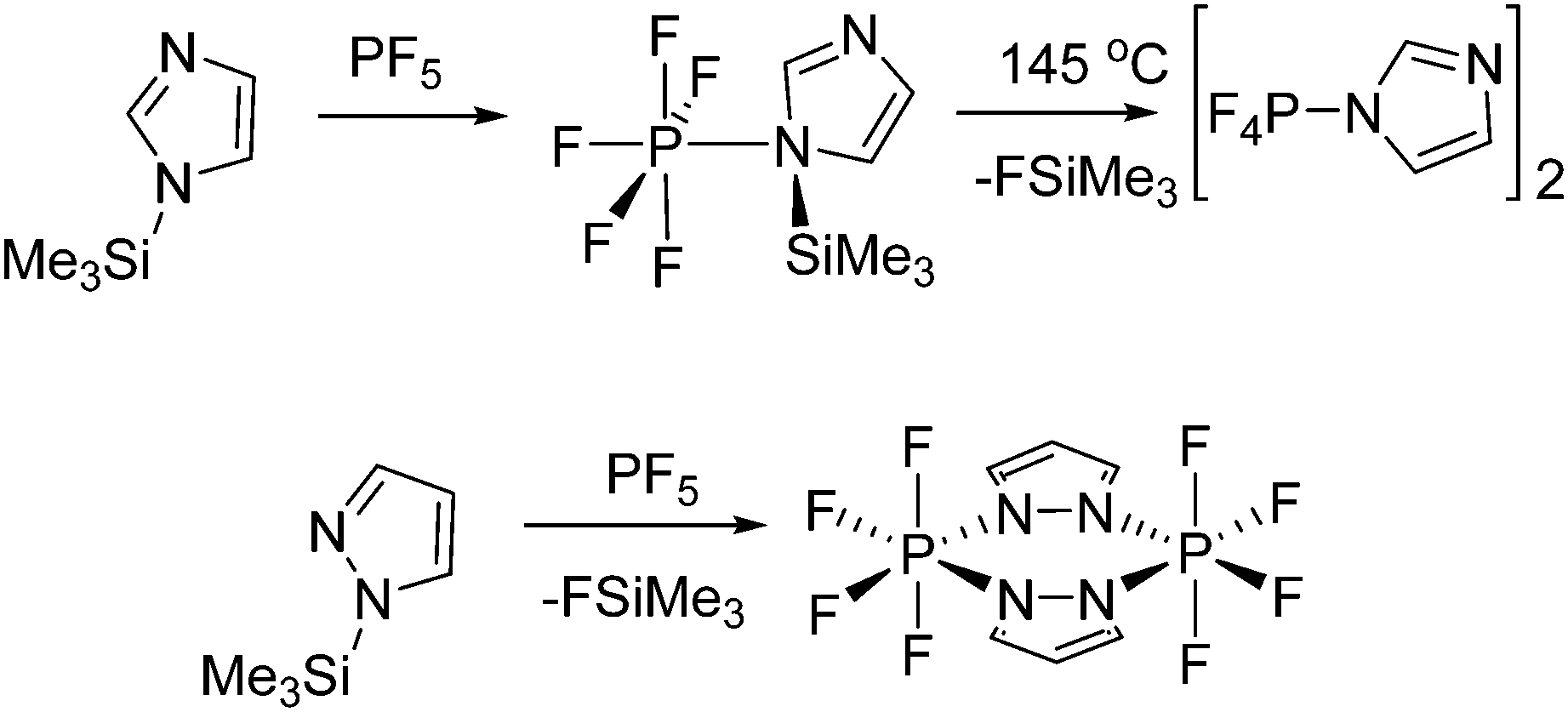
Phosphorus Lewis Acids Emerging Reactivity And Applications In Catalysis Chemical Society Reviews Rsc Publishing Doi 10 1039 C5cs00516g
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora
Welcome To Chem Zipper Com Lewis Acid Base Concept
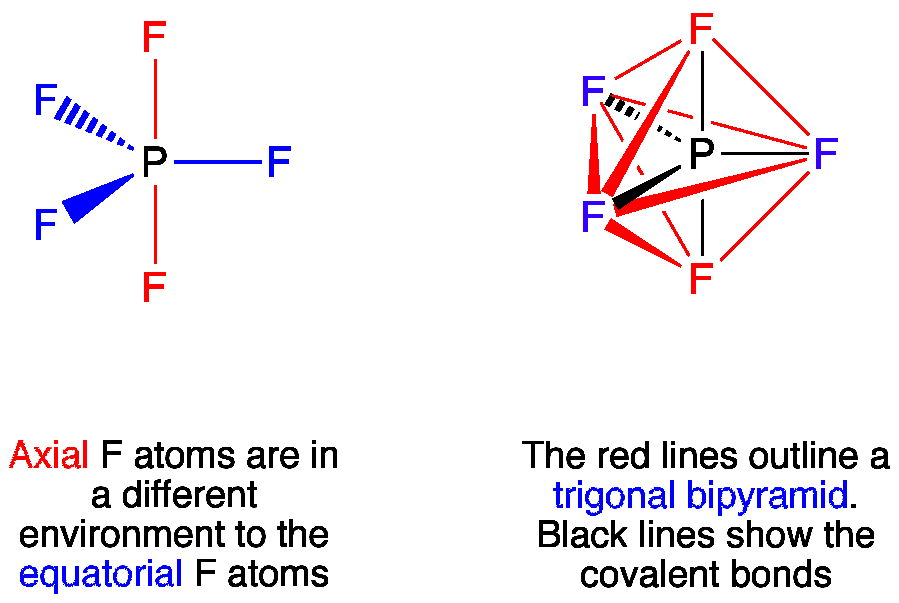
Vsepr Pf5 Phosphorus Pentafluoride

