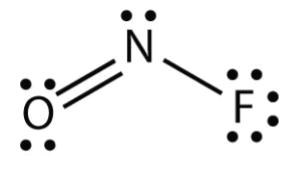
Nof Lewis Structure
Concepts and reason A Lewis structure is a diagram that depicts the chemical bonding between atoms and represents the lone pair of electrons present on the atom. Lastly there is a single unpaired electron on the nitrogen atom.

Draw The Main Lewis Structure Of Nof Draw Clutch Prep
In this case Nitrogen has 5 valence electrons Oxygen has 6 and Fluorine has 7 and we get 18 when we add them up.

Nof lewis structure. Draw the main Lewis structure of NOF. To determine the lewis structure we take the valence electrons of each atom. Since Nitrogen is the center of the structure we now have to place Oxygen.
The lone pair of electrons and bond pairs of electrons are represented on each atom of the Lewis structure. In NOF Lewis structure nitrogen N is the least electronegative atom. A Lewis structure for NO would look like.
The double bar between the two chemical symbols means that nitrogen and oxygen share a double bond2 pairs of electrons. Nitric oxide is composed of a single nitrogen atom that is bonded to a nitrogen atom. How the molecule might react with other molecules.
The central atom is nitrogen since it is the least electronegative atom among. In the Lewis structure for NOF there are a total of 18 valence electrons. Nitrogen trifluoride CH 2 Cl 2 CH 2 S carbon monoxide sulfur trioxide 1 Count all valence electrons 2 Lay out electrons and link with single bonds HONC 3 Attach atoms with pairs of electrons to complete OCTET starting with terminal atoms 4 Complete OCTET for central atoms building multiple.
Draw the main Lewis structure of NOF. NOF Lewis structure. In the lewis structure there are totally 18 valence electrons.
It is also called as a dot structure that represents bonding and non-bonding pairs of electrons in the molecule. NOF also fluorinates fluorides to form adducts that have a salt-like character such as NOBF 4. Determine the number of bonding electrons and the number of nonbonding electrons in the structure of BeF 2.
The Lewis structure for the NOF rm NOFNOF molecule is depicted above and it is checked by finding the formal charges for each atom. Nitrogen has five valence electrons. Fluorine has seven valence electrons.
Nitrosyl fluoride FNO CID 123261 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure.
Get the free Lewis structure widget for your website blog Wordpress Blogger or iGoogle. Drawing the Lewis Structure for NOF In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure. A step-by-step explanation of how to draw the NOF2 Lewis Dot Structure Nitryl fluoride For the NOF2 structure use the periodic table to find the total num.
Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Lewis Structures are important to learn because they help us predict. Get the free Lewis structure widget for your website blog WordPress Blogger or iGoogle.
See full answer below. From the Periodic table. Check the formal charges to be sure that each atom has a formal charge of zero.
Check the formal charges to be sure that each atom has a formal charge of zero. Lewis structure for nof. In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure.
The shape of a molecule. The Lewis structure of eqNOFeq nitrosyl fluoride is given below. Do not get confused while.
The physical properties of the molecule like boiling point surface tension etc. In NOF Lewis structure nitrogen N is the least electronegative atom and goes in the centre of the Lewis structure. Total 18 valence electrons.
Find more Chemistry widgets in WolframAlpha. Oxygen has six valence electrons and. The NOF Lewis structure is very similar to NOCl and NOBr.
Determine the number of bonding electrons and the number of nonbonding electrons in the structure of BeF2. The double bar between the two chemical symbols means that nitrogen and oxygen share a double bond2 pairs of electrons. Lewis structure of NO 2-ion is drawn in this tutorial.
A step-by-step explanation of how to draw the NOF Lewis Dot Structure Nitrosyl fluorideFor the NOF structure use the periodic table to find the total numb. Drawing Lewis Structures Step by step. Draw the main Lewis structure of NOF.

Oneclass Three Possible Lewis Structure For Nof Are Shown Below A Calculate Formal Charges For Eac

Draw The Main Lewis Structure Of Nof Draw Nonbonding Electrons Using The Dot Notation And Bonding Brainly Com
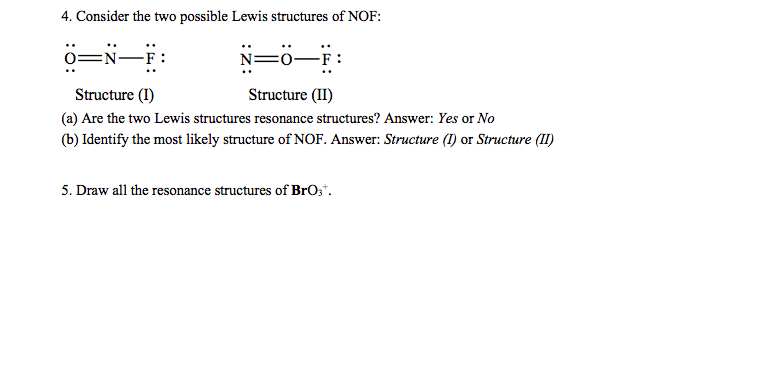
4 Consider The Two Possible Lewis Structures Of Nof Chegg Com

1 Determine The Number Of Bonding Electrons And The Number Of Nonbonding Electrons In The Structure Of Co 2 2 Draw The Main Lewis Structure Of Nof 3 Determine The Number Of Bonding
No2f Lewis Structure How To Draw The Lewis Structure For No2f Youtube
Draw The Main Lewis Structure Of Nof
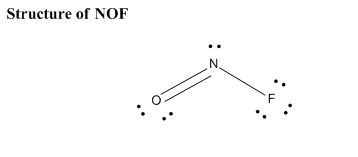
Draw The Main Lewis Structure Of Nof Draw Nonbonding Electrons Using The Dot Notation And Bonding Electrons As A Bond Determine The Number Of Bonding Electrons And The Number Of Nonbonding Electrons

Draw The Main Lewis Structure Of Nof Draw Nonbonding

Draw The Lewis Structure Of Nof Nitrosyl Fluoride Youtube

Nof Lewis Structure How To Draw The Dot Structure For Nof Chemical Bonding
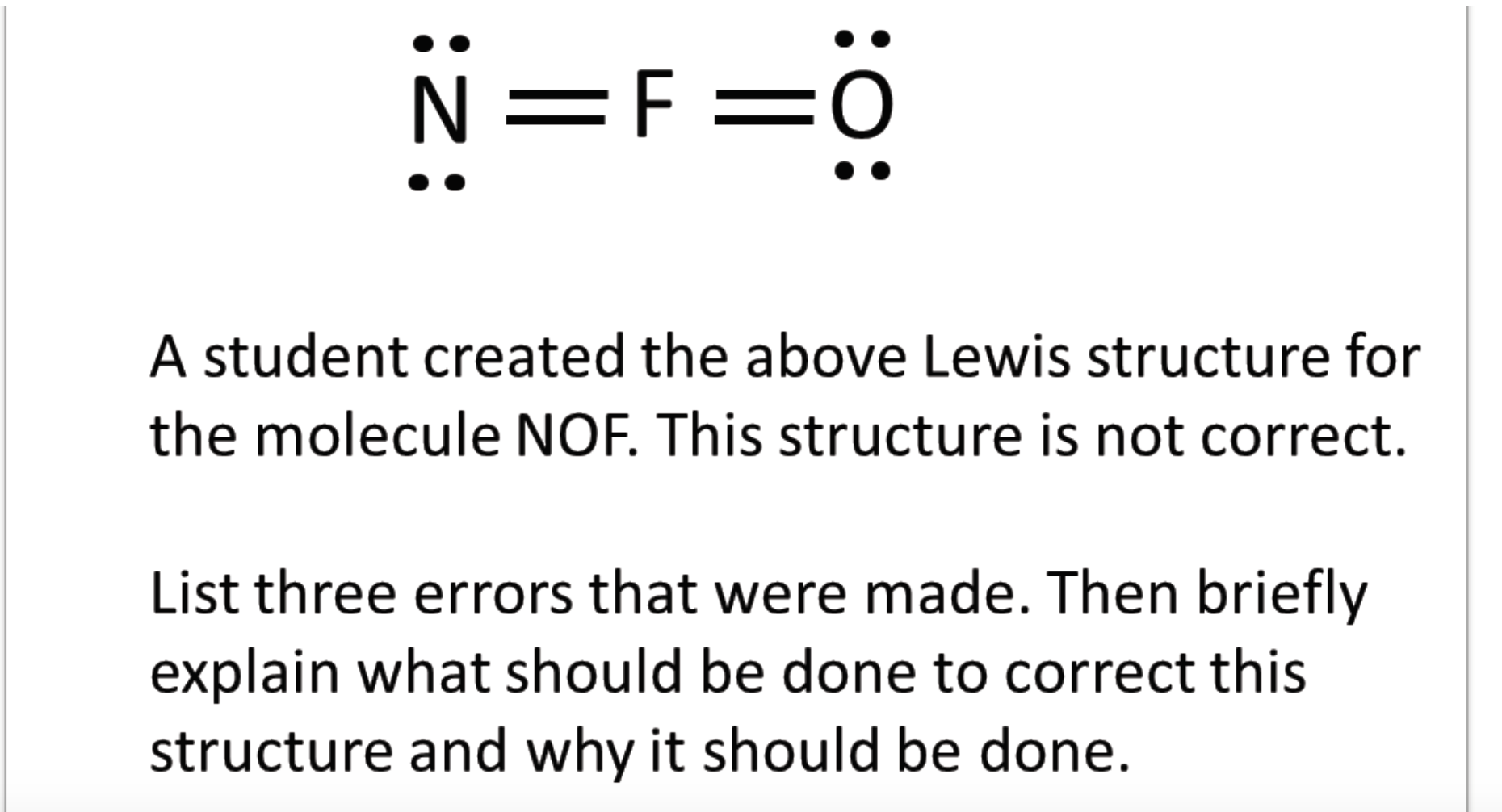
N F 0 A Student Created The Above Lewis Structure Chegg Com
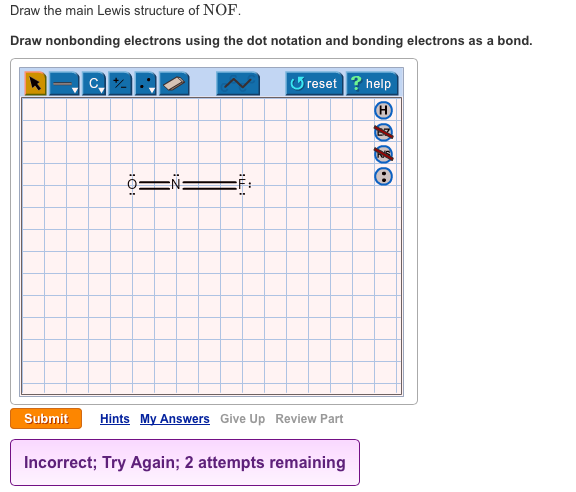
Draw The Main Lewis Structure Of Nof Draw Chegg Com

A Nof B Clf 2 Cl Chlorine Draw The Lewis Structures For The Given Formulas Include Any Resonance Structures If More Than One Lewis Structure Can Be Drawn Use Formal Charges To
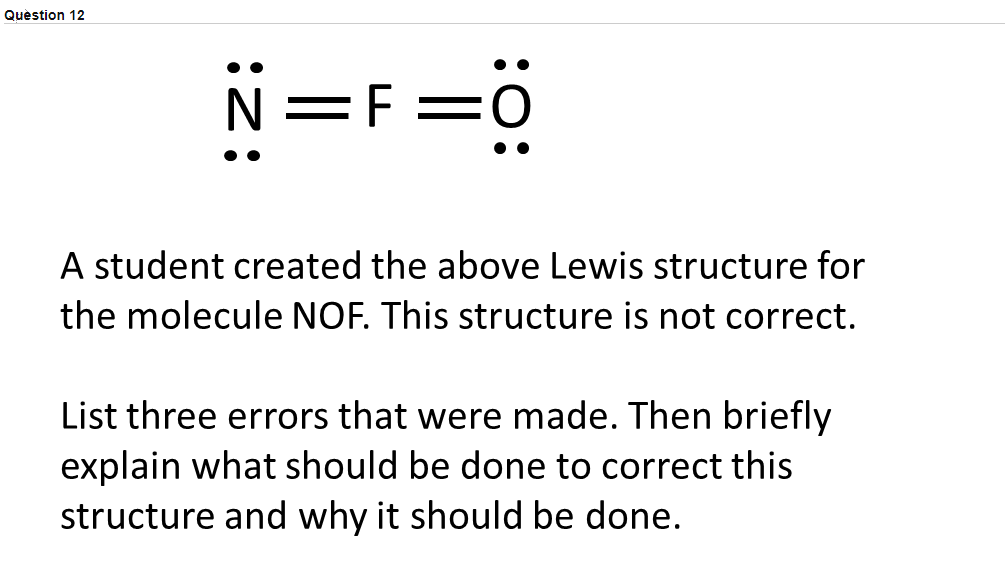
Question 12 N F 0 A Student Created The Above Lewis Chegg Com
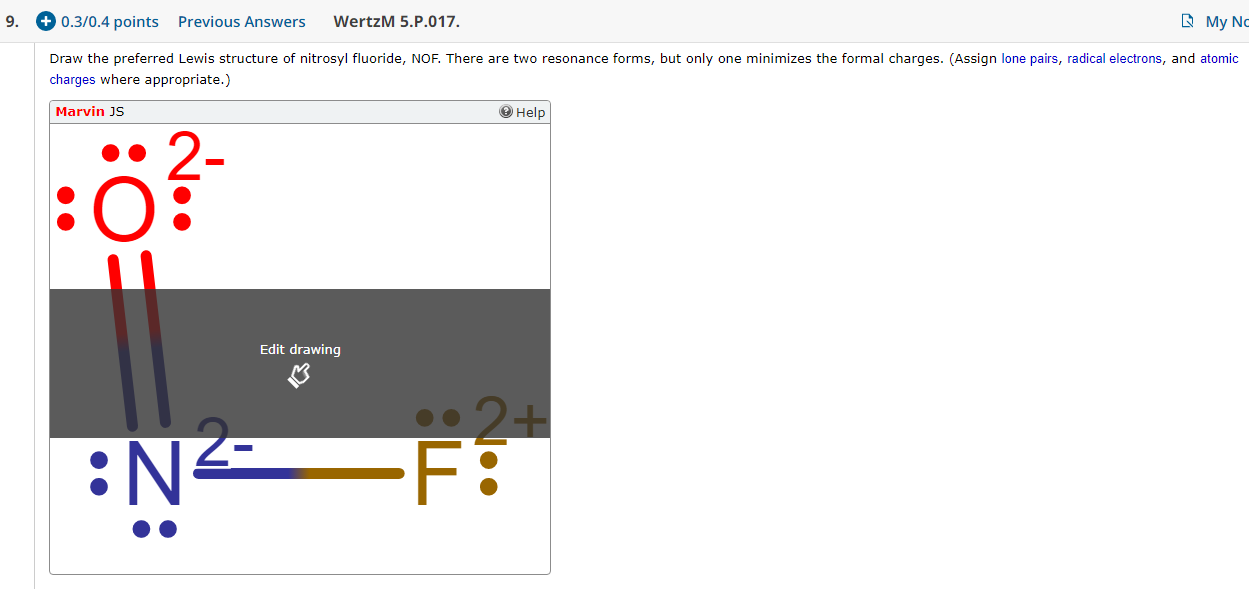
9 0 3 0 4 Points Previous Answers Wertzm 5 P 017 Chegg Com

Nof Lewis Structure Learn Lif Co Id

Nof Lewis Structure How To Draw The Dot Structure For Nof Homeworkavid