Is Pcl5 Polar
This problem has been solved. In PCl5 phosphorus forms 5 polar bonds with cl atoms out of which the two axial bonds have more Bond length than the other three existing equatorial Bond lengths.

Covalent Bonds Types Of Chemical Formulas For Dummies Covalent Bonding Chemical Bond Chemistry Gifts
Therefore if you check chemical structure of PCl5 below then it is clear PCl5 is a nonpolar substance.

Is pcl5 polar. In order for a molecule. What type of intermolecular force is methanol. Hey Guys In this video we are going to determine the polarity of Phosphorus Trichloride having a chemical formula of PCL3To know the polarity of this molec.
As mentioned above one must consider the difference in the electronegativities of the two bonded atoms. Which of the following. Using periodic trends arrange the following molecules in order of increasing dipole moment.
In PCl3 there are also dipole-dipole forces and dipole-induced dipole forces. While the Chlorine atoms are the same there is an Oxygen atom. PCl5 has symmetric charge distribution of Chlorine atoms around the central atom Phosphorous.
Pcl5 is symetrical in shape therefore it is non polar. A PCl3 is polar while PCl5 is nonpolar. Why PCl5 is non polar.
PCl3 is a polar molecule because of its tetrahedral geometrical shape having a lone pair on Phosphorus atom and the difference between the electronegativity of Chlorine316 and Phosphorus219 atoms resulting in unequal sharing of electrons and develop positive and negative poles across the molecule making it a polar molecule. As such the only intermolecular forces active in PCl5 are induced dipole-induced dipole forces London dispersion forces. Does PCl5 have dipole-dipole forces.
As charge distribution is equal and there is no net dipole moment therefore PCl5 molecule is nonpolar. Therefore if you check chemical structure of pcl5 below then it is. However there is no dipole moment between phosphorus atom and chlorine atom.
If you look at the Lewis structure for POCl3 we can see that it is not a symmetrical molecule. In this chemical structure Cl is more electronegative than P. Yes pcl5 is quite polar.
How else could it be a solid melting higher that 160. This problem has been solved. What is the point group of HCl.
Answer PCl5 PHOSPHORUS PENTACHLORIDE is Polar What is polar and non-polar. It has two lone pairs. Linear molecules with a centre of symmetry such as H2 CO2 3 and HCCH belong to D h.
But as the difference is more than 05 PCL3 is polar. Many students may have a query about whether pcl5 is polar or not. In pcl5 phosphorus forms 5 polar bonds with cl atoms out of which the two axial bonds have more bond length than the other three existing equatorial bond lengths.
A molecule that is linear but has no centre of. The LPs always go in the equatorial position in this EG bc there is more space Sif4 Polar Or Nonpolar.

No3 Lewis Structure How To Draw The Lewis Structure For No3 Chemistry Science Chemistry Chemistry Help

Enkelvoudige Ionen Samengestelde Ionen

Enkelvoudige Ionen Samengestelde Ionen

How The Molecular Dipole Moment Affects The Physical Properties Molecular Physical Properties Physics
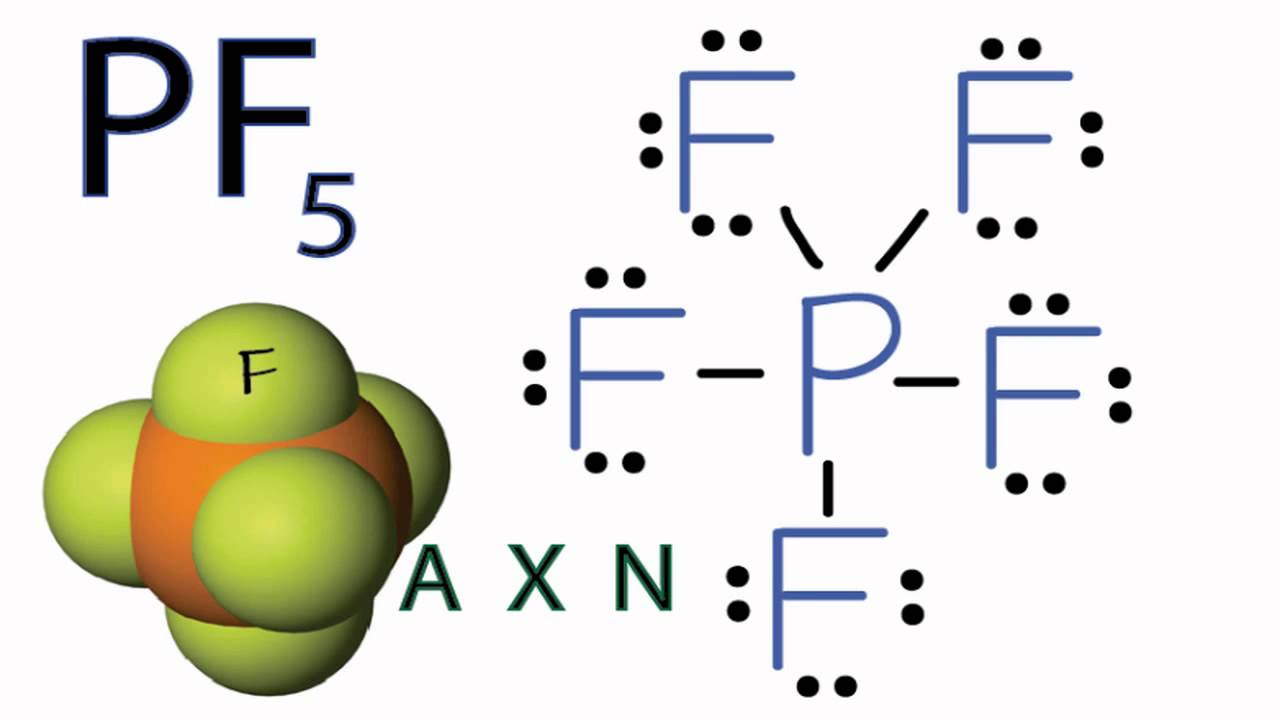
Pf5 Molecular Geometry Shape And Bond Angles Molecular Geometry Geometry Shape Molecular

Ncert Solutions For Class 11 Chemistry Chapter 7 Equilibrium Cbse Tuts 11th Chemistry College Chemistry High School Chemistry

How The Molecular Dipole Moment Affects The Physical Properties Molecular Physical Properties Physics

Simple Procedure For Writing Lewis Structures For Methanimine Ch2nh 25 Chemistry Net Writing Chemistry Math Equations

Simple Procedure For Writing Lewis Structures For Methanimine Ch2nh 25 Chemistry Net Writing Chemistry Math Equations

How The Molecular Dipole Moment Affects The Physical Properties Molecular Physics Chemistry

Dipole Moment Bond Moment Group Moment And Influence Of Dipole Moment Chemsolve Net Organic Chemistry Books Covalent Bonding Physical And Chemical Properties

Dipole Moment Bond Moment Group Moment And Influence Of Dipole Moment Chemsolve Net Organic Chemistry Books Covalent Bonding Physical And Chemical Properties
