What Is The Bond Angle Of C2h4
5 rows There is a formation of a sigma bond and a pi bond between two carbon atoms. The diagram below shows the bond lengths and hydrogen-carbon-carbon bond angles of ethene.

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
The term sp3 hybridization refers to the mixing character of one 2s-orbital and three 2p-orbitals to create four hybrid orbitals with similar characteristics.

What is the bond angle of c2h4. A Less energy is released on bond making than is taken in during bond breaking. See the title structure below Flat but I cant see anything from the angle. It turns out that methane is tetrahedral with 4 equal bond angles of 1095 and 4 equal bond lengths and no dipole moment.
Why the ethylene molecule is perpendicular to the PtCl3 molecular plane in Zeises salt. The bond angle of C2H4 is 120º. And there is no free rotation about a carbon-carbon double bond.
The C on the other hand has atoms no lone pairs so it. Molecular geometry This is ethane an alkyne double H to H with 2 carbon atoms which means that the relationship between the carbon atoms is double. Each H-C-H bond angle is around 1173.
Here in C2H4 both carbons are sp2 hybridized so they are planar with bond angle of 120degree. Determine the molecular geometry about each interior atom and make a sketch. They say both shape and angle here.
Is C2H4 a tetrahedral. This is composed of a σ framework and a π-bond. What Is The H-C-C Bond Angle In C2H4.
C2H6 is sp3 hybridised so form Tetrahedral geometry which has all H hydrogen out of plane hence non planar. Well determine the N2H4 molecular geometry with respect to the Nitrogen on the right the other Nitrogen atom will have the same shape since they are symmet. C2H4 has the Lewis Structure.
The bond angle is around 120 degrees. Which intermolecular forces exist between molecules of CO. The first and foremost thing that we need to look into while finding out the hybridization of any.
In order for the unhybridized p orbitals to successfully overlap the CH 2 must be coplanar. Group Of Answer Choices About 180 Degrees About 90 Degrees About 1095 Degrees About 120 Degrees This problem has been solved. Each C-H bonds are of equal length Ie.
Is becl2 resonance structure. This means that the. C2h4 Molecular Geometry What is the shape of the molecule and the binding angle of C2H4.
C2H4 Bond Angles According to the VSEPR theory the Hydrogen atoms on both Carbon atoms will repel each other giving rise to a bond angle of 1213. Each HCH bond angle is around 1175º because the presence of a double bond in between carbon atoms shrinks the angle between the HCH bond from 120º to 1175º. This is smaller than trigonal planer angle of 120 because the pi cloud over CC inserts a repulsion on bond pairs and hence contracts the ideal angles.
C2H4 Hybridization Atomic orbitals combine together to form hybrid orbitals and the process is known as hybridization. 1087 pm and CC bond is 1339 pm long smaller than C-C single bond as pi bonds cause shortening of bond length. Secondly what does c2h4 look like.
B The enthalpy change for the reverse equation is 1846 kJmol-1 c The enthalpy change of formation of HCl g is -1646 kJ mol-1 d The temperature decrease during the reaction. Describe the bonding present in the ammonium ion NH4 Sharing of electrons between atoms. As a consequence the C C bond length in Zeises salt increases from free C2H4 molecule 134 Å to coordinated C2H4 molecule 14 -147 Å.
Accordingly what is the bond angle of c2h2. State the shape of the molecule and its bond angles in NH3. C 2 H 4 Molecular Geometry.
The Lewis structure of BeCl2 is. Hence C2H4 is planar. Therefore C 2 H 4 is a planar molecule and each bond angle is about 120 degrees.
Therefore there cannot be more than one stable resonance structure for C2H4. Resonance structures are used to represent the different possible bonding arrangements in a molecule. And C2H4 has a trigonal molecular geometry so the bond angle will be about 120.
H2CCH2 April 6 2021 by Answerout Here is the answer for the question Determine the molecular geometry about each interior atom and make a sketch. What is methane bond angle. The resonance is formed when an electron pair from each chlorine atom forms a double bond with Be.
The lone pairs around the oxygen repel the bonding electrons such that the bonding angle between atoms is less than 1095. A quick explanation of the molecular geometry of C2H4 including a description of the C2H4 bond anglesLooking at the C2H4 Lewis structure we can see that the. The molecular shape is predicted to be trigonal planar around each carbon atom.
In ethane C2H6 BOnd angle is 1095 in etheneC2H4 bond angle is 120 in ethyne C2H2 Bond angle is 180 MARK AS BRAINLIEST. What is sp3 hybridisation.

How Is C2h4 Planar While C2h6 Is Non Planar Quora

Chemistry Molecular Structure 15 Of 45 Basic Shapes Predict The Shape Of C2h4 Youtube

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Molecular Geometry Shape And Bond Angles Youtube
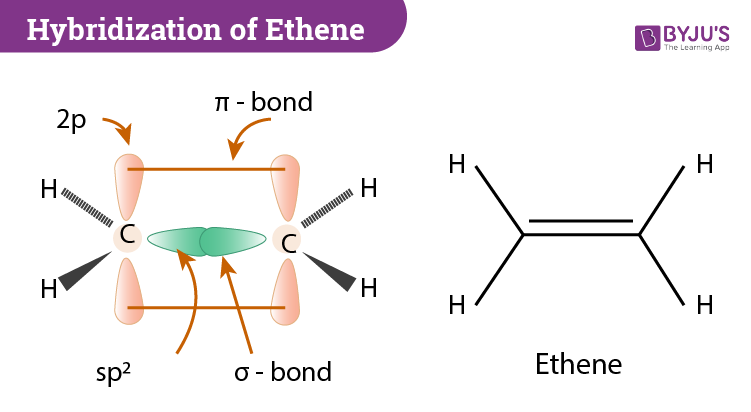
Hybridization Of C2h4 Ethene Hybridization Of Carbon In C2h4

Chemical Bonding Ii Molecular Geometry And Hybridization Of Atomic Orbitals Chapter 10 Copyright C The Mcgraw Hill Companies Inc Permission Required Ppt Download

Structure Of H3bo3 And C2h4 C O 3 Ozone O 3 Exists As A Stable Download Scientific Diagram
How Is C2h4 Planar While C2h6 Is Non Planar Quora

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
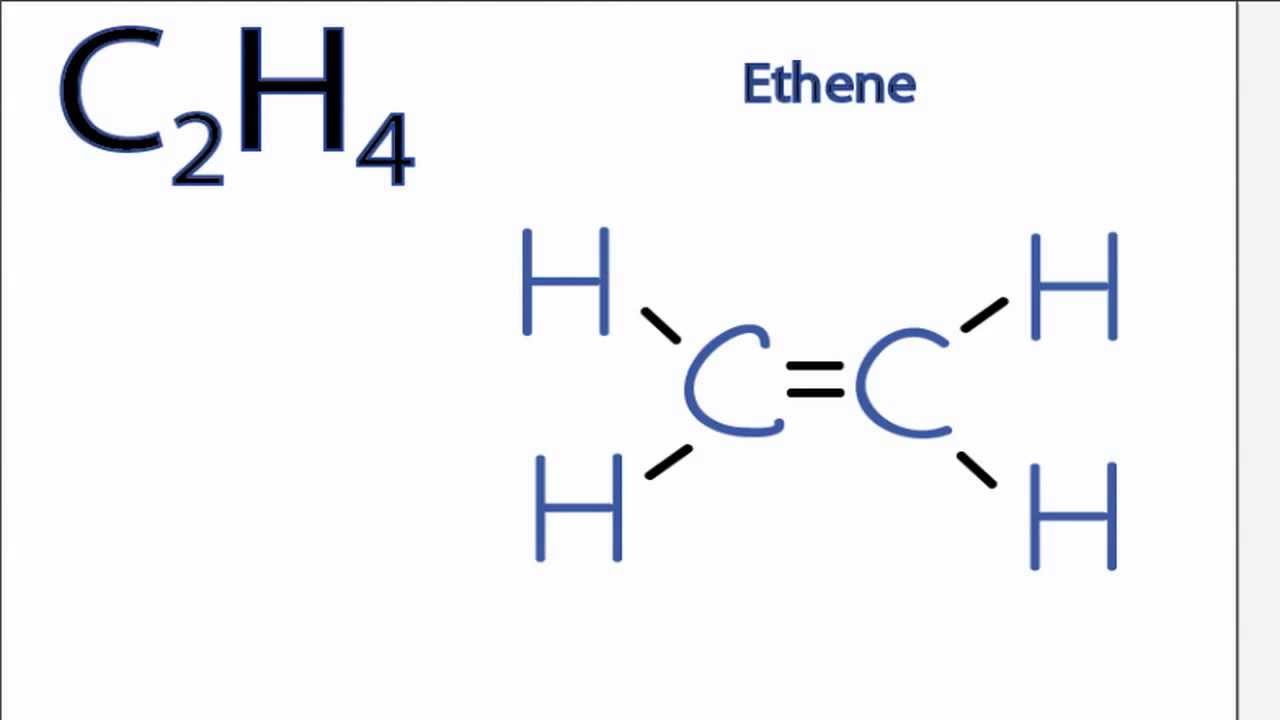
Is C2h4 Polar Or Nonpolar Youtube
Illustrated Glossary Of Organic Chemistry Bond Angle

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
C2h4 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity

How Is The Structural Formula For C2h4 Determined Quora
What Is The Hybridization And Bond Angle Of A C2h4 Molecule Quora

C2h4 Molecular Geometry How To Discuss

Ch 10 Vsepr Practice Problems 3 Flashcards Quizlet