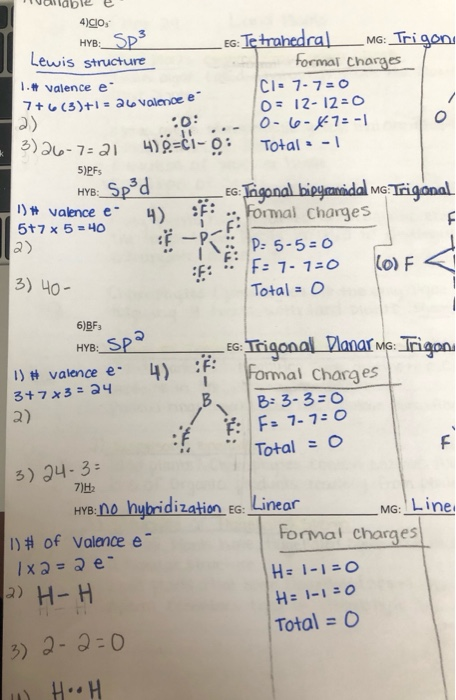
Pf5 Lewis Structure Formal Charge
Formal charge Nve. Type Out Your Calculation.
Can Someone Please Help Me With The Molecular Chegg Com
If you draw a dot structure showing the sulfur atom single bonded to each of the four oxygen atoms the formal charge on each oxygen atom is -1 and the formal charge on the central sulfur atom is 2.

Pf5 lewis structure formal charge. B A plausible Lewis structure for N2H5 is given below. In OF2 however oxygen is the central atomUse formal charges. FC V N B Where.
Explain why or why not. C Hydrogen peroxide is sold commercially as an aqueous solution in brown bottles to protect it from light. The formal charges must add to the overall charge of the ion.
A Draw the Lewis structure for hydrogen peroxide. Draw lewis strucutre of the following molecules and ions. Determine the central atom in the molecule.
PF5 Lewis Structure - How to Draw the Lewis Structure for PF5 Phosphorus Pentafluoride. Some practice of assigning formal charge is necessary before you master this technique. Five pairs will be used in the chemical bonds between the P and F.
Explain Why Or Why Not. NO31- SO3 PF5 HCN N2O SF4 which of them is are polar. B What is the weakest bond in hydrogen peroxide.
Calculate the formal charge for the indicated atom. And what are hybridization for all of them. Consider the molecule PF5.
Is The Octet Rule Obeyed For Every Atom In The Structure. We know from the previous post that the formal charge can be calculated by this formula. The formal charges in a structure tell us the quality of the dot structure.
In the PF 5 Lewis structure Phosphorus P is the least electronegative so it goes in the center. In a Lewis structure formal charges can be assigned to each atom by treating each bond as if one-half of the electrons are assigned to each atom. One s orbital three p orbitals.
The formal charge is zero on each atomchlorine and fluorine in the ClF3 Lewis structure. A A plausible Lewis structure for the nitrosonium cation NO is drawn below. Calculate the formal charge for Pin PF5.
Egtrigonal bipyramidal mgtrigonal bipyramidal Draw the best Lewis structure for BrO4- and determine the formal charge. A step-by-step explanation of how to draw the PF5 Lewis Dot Structure Phosphorus PentafluorideFor the PF5 structure use the periodic table to find the tot. Ii how many sigma and pi bonds are there in No2.
These hypothetical formal charges are a guide to determining the most appropriate Lewis structure. Calculate the Formal Charge of Oxygen on the Right. Consider The Molecule PF5.
The formal charge of oxygen left is 0. Oxygen O is in group 16 so that means it has 6 valence. Thus we calculate formal charge as follows.
In N2O nitrogen is the central atom and the oxygen atom is terminal. Type out your calculation. Thus 4 -1 2 -2.
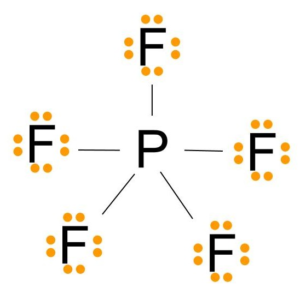
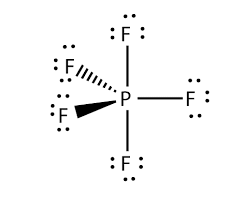
There are no lone pairs in the Lewis Structure of PF 5 and there are five single bonds between Phosphorus and Fluorine atoms. The formal charge is a hypothetical charge assigned from the dot structure. Solved Nov 12 2018.
Calculate The Formal Charge For F In PF5. This gives each atom an octet and a positive formal charge appears on the oxygen atom. Formal charge 6 - 4 - 42 0.
The Lewis Structure Is Shown Below. Is the octet rule obeyed for every atom in the structure. Formal charge of each F atom Valence electrons 7 12Bonding electrons 2 Lone pair of electrons 23 7 1 6 0.
Calculate the longest wavelength of light that has sufficient energy to break the weakest bond in hydrogen peroxide. There are five half-filled orbitals. How Many Valence Electrons Are In The Molecule PF5.
Formal charge alencev shell electrons free atom lone pair electrons 1 2 bonding electrons 1 We can double-check formal charge calculations by determining the sum of the formal charges for the whole. Use formal charge to explain why the fulminate ion is less stable and therefore more reactive than the cyanate ion. Calculate The Formal Charge For P In PF5.
Calculate the total number of valence electrons present. Were being asked to determine the formal charge of ALL ELEMENTS in PCl3 and PF5. Determine the electronic geometry eg and molecular geometry mg of PF5.
In the Lewis structure for PF 5 there are a total of 40 valence electrons. The electron configuration of a Phosphorus atom in its ground state is 1s2 2s2 2p6 3s2 3p3 but when it is in an excited state the electrons from 3s orbital get unpaired. The Lewis structure is shown below.
A structure in which the formal charges are as close to zero as possible is preferred. Electrons and then subtract the number of bonds connected to that atom in the Lewis structure. N O The nitrogen atom is triply bonded to the oxygen atom and both atoms in the structure possess a lone pair of electrons.
Draw the Lewis structure for the molecule. To do so we need to do the following steps. How many valence electrons are in the molecule PF5.
As lower the formal charge higher is the stability of lewis diagram. V number of valence electrons N number of nonbonding electrons B number of bonds Notice that this is not the only formula for calculating the formal charge however I figured it was the best variation acceptable to my students. Show all formal charges.
Calculate the formal charge for Fin PF5.

Pf5 Lewis Structure Phosphorus Pentafluoride Youtube

Chemical Bonding I Basic Concepts Chapter 9 Copyright
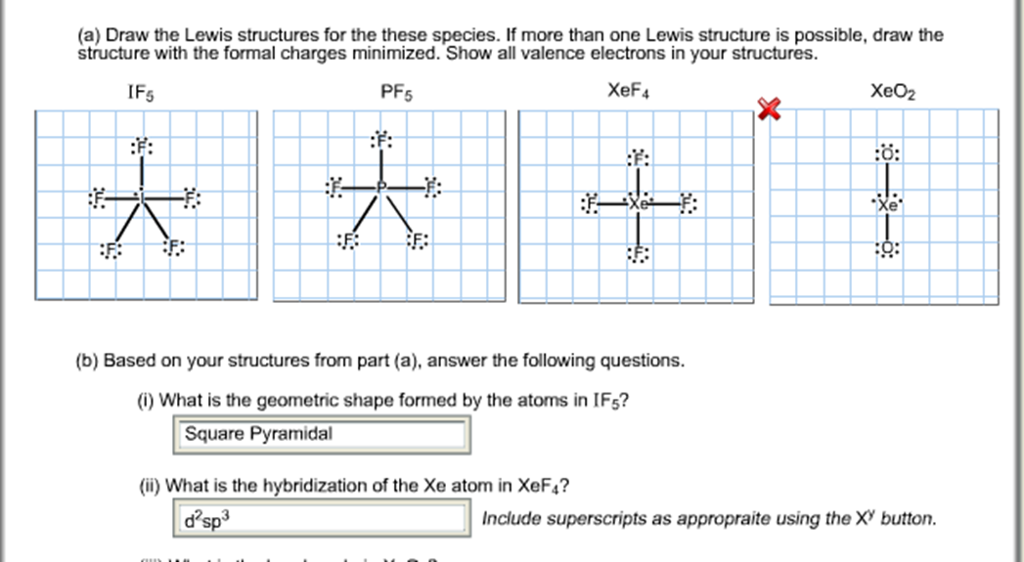
A Draw The Lewis Structures For The These Species Chegg Com

Pf5 Lewis Structure And Molecular Geometry Made Easy Youtube

Chemical Bonding I Basic Concepts Copyright The Mc
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules

Pf5 Lewis Structure Phosphorus Pentafluoride Youtube

Calculate The Formal Charge Of Each Elemen Clutch Prep
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules

Consider The Molecule Pf5 The Lewis Structure Is Chegg Com
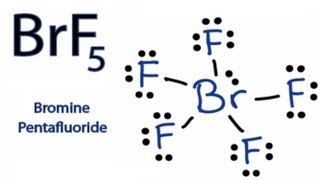
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube
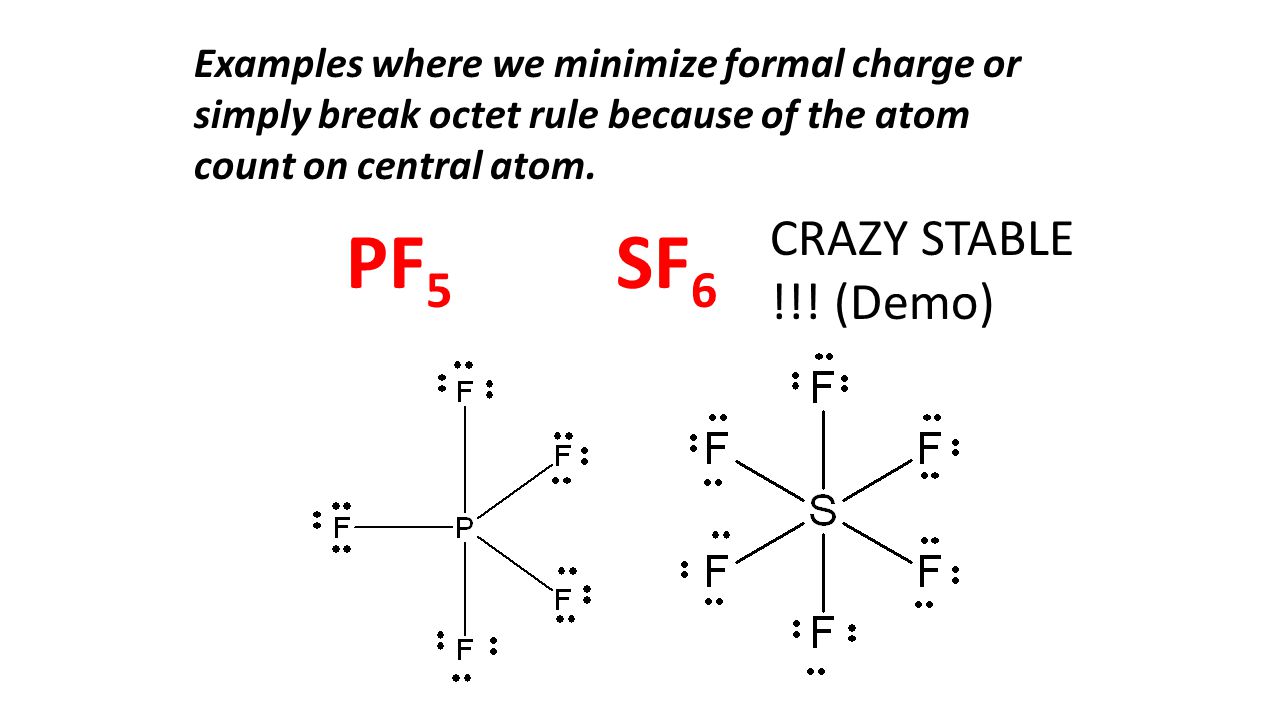
Octet Rule Structures Ppt Video Online Download

Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
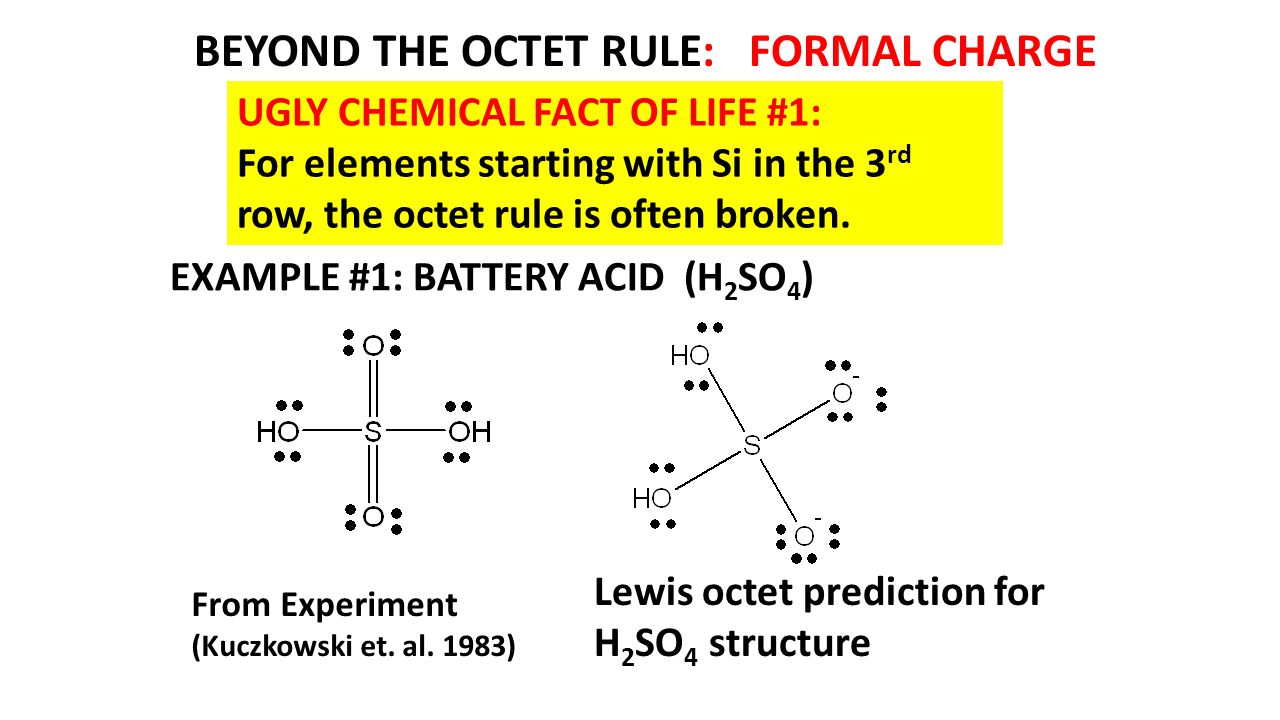
Octet Rule Structures Ppt Video Online Download

Xeo4 Lewis Structure How To Draw The Lewis Structure For Xeo4 Youtube

Molecular Geometry Of Pf5 Phosphorus Pentafluoride Youtube
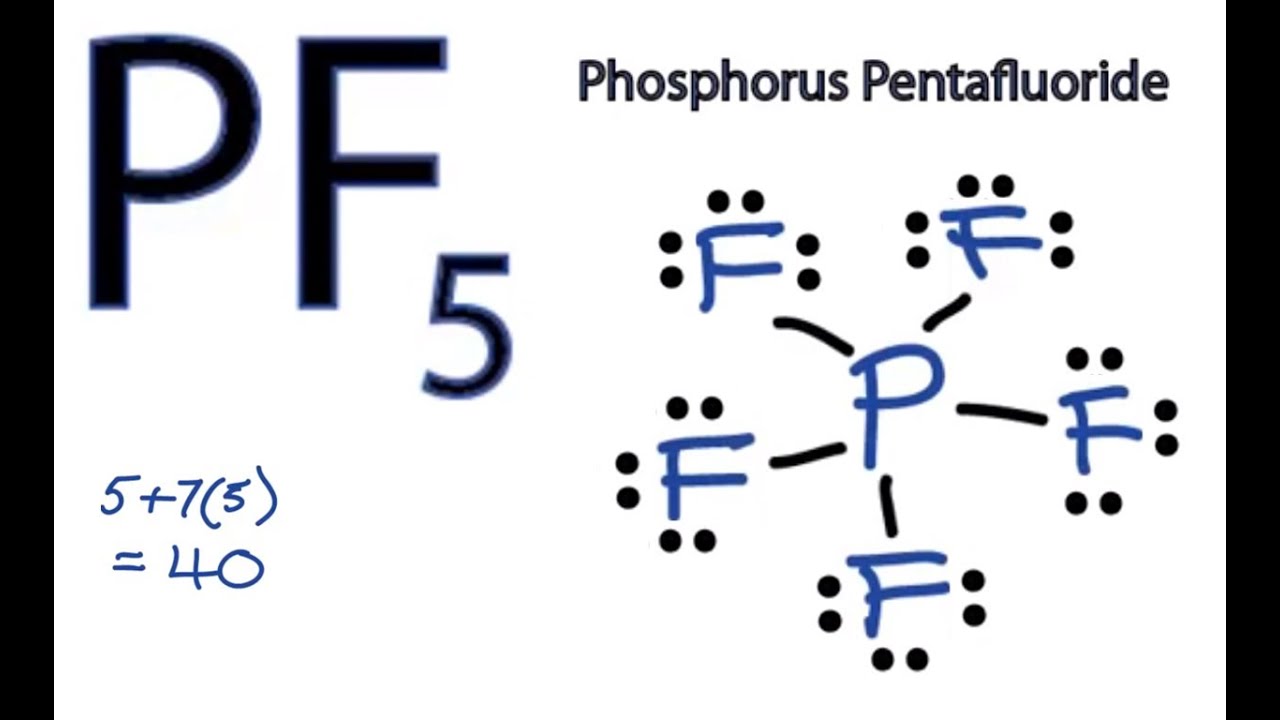
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Pf5 Lewis Structure And Molecular Geometry Made Easy Youtube