How Many Lone Pairs Are In C2h2
The carbon atoms are bonded to each other and each hydrogen atom is bonded to a carbon atom. Other examples include CO2 HCN C2H4 C2H2.

C2h2 Molecular Geometry Shape And Bond Angles See Description For Note Youtube
Find the number of nonbonding lone pairs e-.

How many lone pairs are in c2h2. C2H2 Molecular Geometry And Bond Angles As a result of the double bond C2H2 molecular geometry is linear with a bond angle of 180o. How many lone pair in C2H2. Chemical Nameformula Valence Electrons Hybridi.
Add your answer and earn points. The C2H2 molecule contains a triple bond between the two carbon atoms one of which is a sigma bond and two of which are pi bonds. Hydrogen has one bond and no lone pairs.
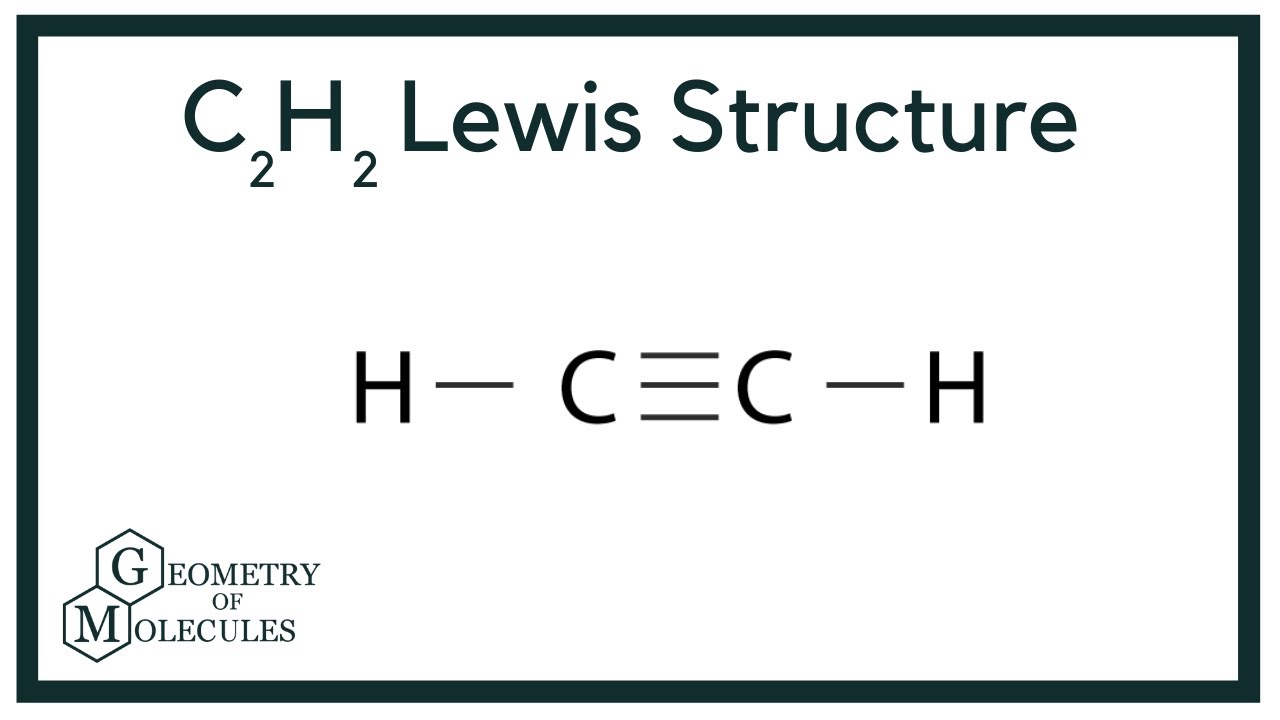
The Lewis structure for C2H2 contains. There are no lone pairs so this is also the molecular geometry. There are no lone pairs on carbon or hydrogen atoms.
B For this compound Identify the following -number of electron groups electron domains -number of atoms bounded to the central atom -number of non-bounding electron pairs lone pairs attached to the central atom -General formula c What is the. C2H2 a How many lone pairs non-bounding electron pairs does the compound possess on All atoms. Central atoms and outer atoms.
There is a triple bond between carbon atoms. Fok2581 is waiting for your help. A how many single bonds b how many double bonds c how many triple bonds and d how many lone pairs.
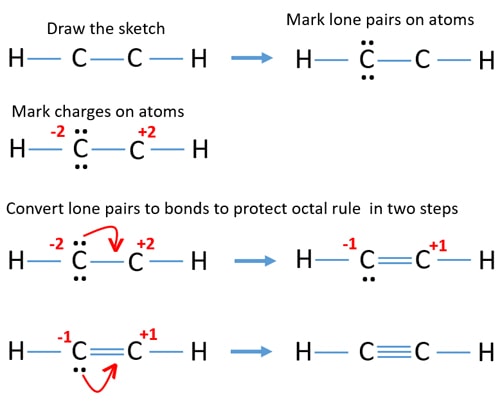
In this tutorial we are going to learn how to draw lewis structure of C2H2. There can be a maximum of three bonds between 2 Cs. Include all lone pairs of electrons.
The Lewis structure for C2H2 contains. C2H2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. In the structure of C2H2 you can see that each of the 2 Cs have 4 bonds each C having 1 with HydrogenH and remaining 3 with the other C.
The O in HOCl has two lone pairs and two bonding pairs in a tetrahedral arrangement which is sp3. Which of the following compounds contains exactly one unshared pair of valence electrons. How many total bonds and lone pairs exist in the Lewis structure for chlorine fluoride ClF.
What type of bond does c2h2 have. How many types of law are there in physics. A how many single bonds b how many double bonds c how many triple bonds and d how many lone pairs.
Arrange electrons until both carbon and nitrogen get a triple bond giving an octet and hydrogen has 2. B CH2O There are 4 valence electrons in carbon 1 each in hydrogen and 6 in oxygen so there are 12 electrons total. Geometry is trigonal planar and because there are no lone.
How many valence electrons are in lone pairs in the Lewis dot structure of C2H2. This video tutorial compares the features of a bonding pair of electrons to a lone pair of electrons and how those features affect the overall geometry of a. There are 2 bond pairs between carbon atoms and 4 bond pairs between carbon and hydrogen atomsNo lone pair.
Use information from step 4. What is the bond order of the CC bond in acetylene ethyne C2H2. There are no lone pairs in the central atom of boron trichloride because it is one of the exceptions in the octet rule.
Draw the best Lewis structure for C2H2 by filling. How many lone pairs are in bcl3. What is the bond order of the CC bond in acetylene ethyne C2H2.
The first bond that a C makes with a C or any other element the bond is said to be sigma-bond. 22-10 12e-6 lone pairs. Put carbon in the center and arrange hydrogen and nitrogen atoms on the sides.
Subtract step 3 number from step 1. Quarterfreelp and 20 more users found this answer helpful. Which of the following compounds contains exactly one unshared pair of valence electrons.
Find number of bonds by diving the number in step 3 by 2 because each bond is made of 2 e- 10e-2 5 bond pairs. Lewis Structures - Dot Diagram Formal Charge Molecular. How many total bonds and lone pairs exist in the Lewis structure for chlorine fluoride ClF.

C2h2 Acetylene Ethyne Lewis Structure

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com

C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle

Showing Covalent Bonding Using Dot Cross Diagrams Ppt Video Online Download
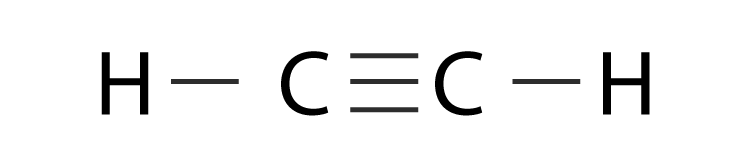
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne
C2h2 Molecular Geometry Shape And Bond Angles See Description For Note دیدئو Dideo

C2h2 Acetylene Ethyne Lewis Structure
C2h2 Acetylene Ethyne Lewis Structure

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

Is C2h2 Polar Or Nonpolar All About C2h2 Polarity

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle

Molecular Geometry Of Acetylene Chemistry Stack Exchange
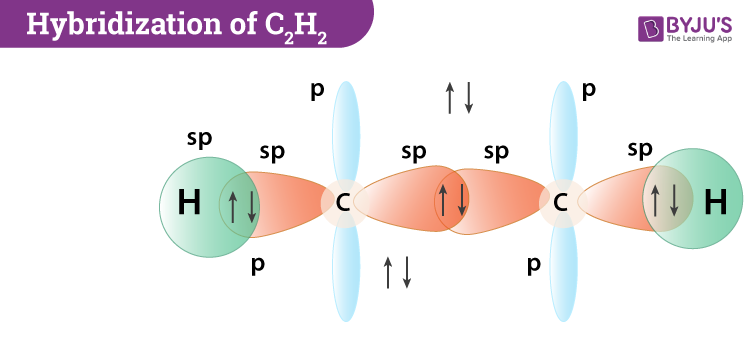
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne

Below Is The Lewis Structure Of The Acetylene C2h2 Chegg Com
Lewis Structure For C2h2 Ethyne

