Ncl4 Lewis Structure
Iodines the least electronegative well put that at the center and then well put four Chlorines around it. Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.

Which Of The Following Molecules Is Likely To Form Note T Clutch Prep
Iodine 7 valence electrons.

Ncl4 lewis structure. Since Iodine I is below Period 3 on the periodic table it can hold more than 8 electrons. And then we have this up here so were going to add an additional valence electron for a total of 36 valence electrons. With five nuclei the ICl4 ion forms a molecular structure that is square planar an octahedron with two opposite vertices missing.
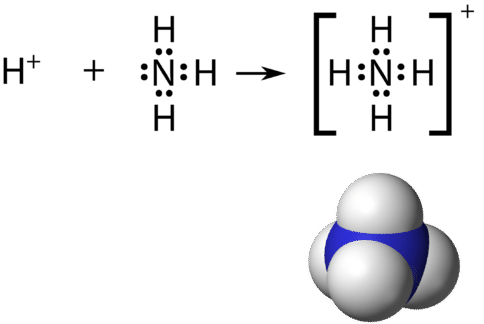
The Lewis diagram from carbon tetrachloride is. Expert Answer 100 1 rating. The Lewis Dot Structure for NH4 Ammonium is shown above.
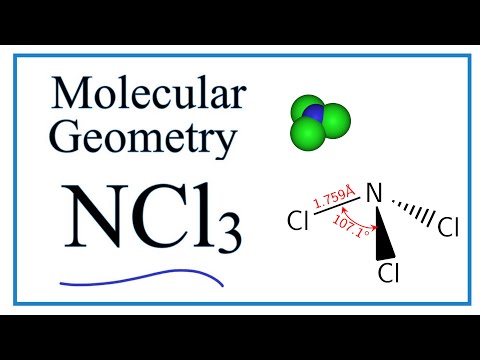
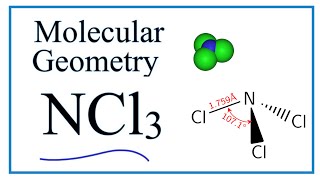
It also mentions the bond angle hybridization and molecular geometry of NCl4 Sih4 Lewis Structure only possible case is that of two double bonds. For ICl4- Draw The Lewis Structure Draw This Structure Indicating The Coordination Geometry Around Iodine And Give An Estimate Of The Bond Angles Is There Resonance In This Compound. Lewis structure of NCl3 can be drawn by using valence electrons of nitrogen and chlorine atoms.
These hypothetical formal charges are a guide to determining the most appropriate Lewis structure. These kinds of structures can also be shown by representing each of the bonds with two dots. 32 valence electrons.
As there are four molecules of Chlorine we will calculate the number of valence electrons accordingly. A bond corresponds to 2 electrons shared between two atoms so we currently have 4 2 e 8 val. It was in 1916 when Gilbert N.
We can add 3 lone pairs to each of the chlorine atoms and then add a lone pair to iodine. S 2 2-OCl-NCl 4 ClO 2-SOCl 2. In a Lewis structure formal charges can be assigned to each atom by treating each bond as if one-half of the electrons are assigned to each atom.
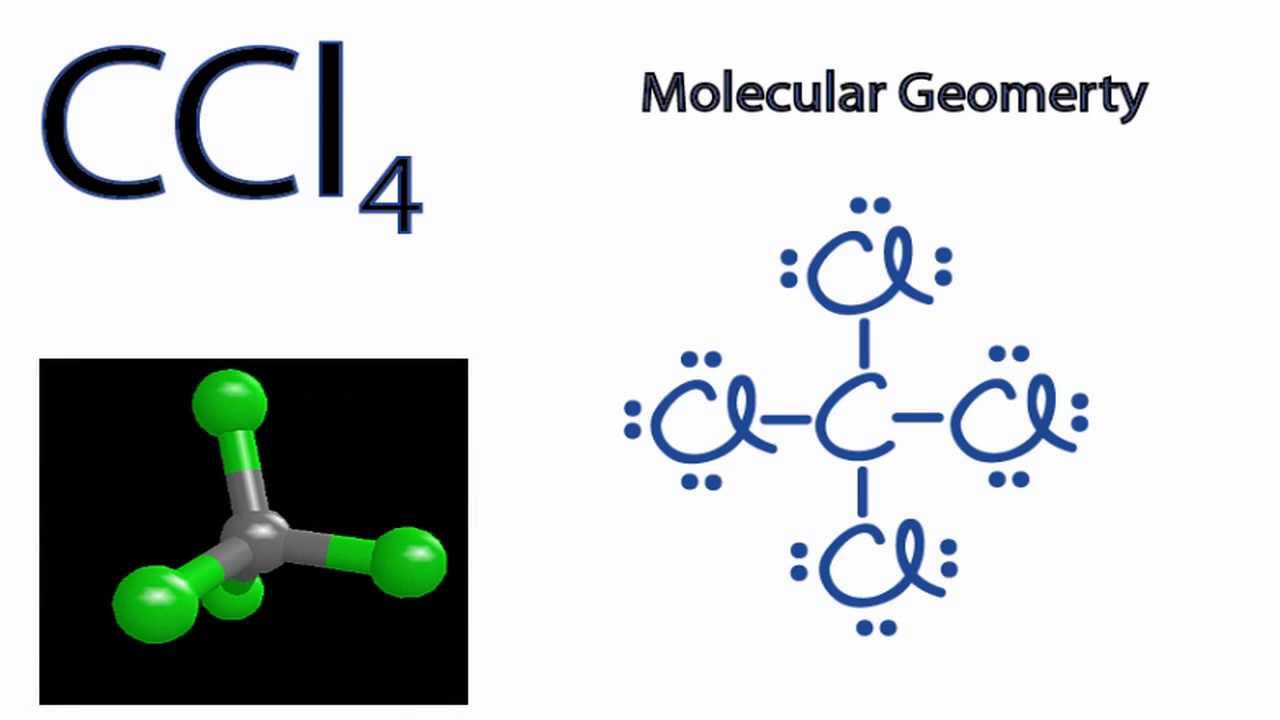
Iodine is bonded to 4 Cl so it forms 4 bonds. For the Lewis structure of CCl4 first lets calculate the total valence electrons. Lewis first introduced the concept of Lewis structures.
The iodine atom has a formal charge of -1 so. Thus it will use its s-orbital its three p-orbitals and one d-orbital mixing them to form five sp3d hybrids. These structures are described as simplified diagrams that visualize how atoms are bonded in.
In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. The relationship between the number of electron groups around a central atom the number of lone pairs of electrons and. There is one lone pair on nitrogen atom and three lone pairs on each chlorine atom.
In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. Chlorine has 7 electrons and so is 1 electron short of completely filling its outer shell. Lets do the ICl4- Lewis structure.
Draw the correct Lewis structures for the following molecules 12 CH 3 F. Steps of drawing the lewis structure of NCl3 are explained in detail in this tutorial. E which means were still lacking 26 e.
Chlorine 7 as well we have four Chlorines. In each case there are five electron pairs around the central atom so it needs fo have five hybrid orbitals to accommodate them. The iodine atom shares 4 valence electrons through 4 single covalent bonds to 4 terminal chlorine atoms.
Well form chemical bonds between the I and the Chlorines. In the Lewis structure of ICl4- there are total of 36 valence electrons. Draw The Correct Lewis Structures For The Following Molecules 12 CH3F S22- OCl- NCl4 ClO2- SOCl2.
The Lewis structure of ICl4 is the same as that for ClF4. Nitrogen trichloride NCl3 lewis structure contains three N-Cl bonds. Each atom in the bond has a full valence shell with nitrogen having access to eight electrons and each hydrogen having access to two this is why hydrogen only needs two.
The incomplete Lewis structure of ICl4 is. Hcl Lewis Structure n2cl4 lewis structure Oct 15 2015 This video shows you how to draw the lewis dot structure of NCl4. Carbon tetrachloride CCl 4 is a covalently bonded compound composed of a central carbon surrounded by 4 chlorine atoms in a tetrahedral structure.
This problem has been solved. A structure in which the formal charges are as close to zero as possible is preferred. A regular atom of carbon has 4 lone electrons in its outer shell.
If So Draw The Resonance Structures. 4 47 4 28. This problem has been solved.
Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA website may be reproduced distributed andor used totally or in part for non-commercial purposes provided that ECHA is. Each chlorine atom shares 1 valence electron. Carbon has four valence electrons and each Chlorine atom has seven valence electrons.
Also there are no charges on atoms in NCl3.
Ccl4 Molecular Geometry Shape And Bond Angles Youtube

Ncl4 Lewis Dot Structure Bond Angle Hybridization Molecular Geometry Quizalize

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Wn Ncl4 Lewis Dot Structure Bond Angle Hybridization Molecular Geometry
Wn Ncl4 Lewis Dot Structure Bond Angle Hybridization Molecular Geometry
Draw The Lewis Dot Structures For These Mulecules Chegg Com

Which Of The Following Molecules Is Likely To Form Note T Clutch Prep

Wn Ncl4 Lewis Dot Structure Bond Angle Hybridization Molecular Geometry
15 4 Lewis Structures Counting Valence Electrons Chemistry Libretexts

Wn Ncl4 Lewis Dot Structure Bond Angle Hybridization Molecular Geometry
Ncl3 Molecular Geometry Shape And Bond Angles Youtube

Which Of The Following Molecules Is Likely To Form Note T Clutch Prep

Calculated Ccsd T Cc Pvtz Geometries Of Ncl 2 Ncl 3 Ncl 4 Download Scientific Diagram

Wn Ncl2 Lewis Dot Structure Molecular Geometry Bond Angle

Which Of The Following Molecules Is Likely To Form Note T Clutch Prep

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Wn Ncl4 Lewis Dot Structure Bond Angle Hybridization Molecular Geometry

Which Of The Following Molecules Is Likely To Form Note T Clutch Prep

Wn Ncl4 Lewis Dot Structure Bond Angle Hybridization Molecular Geometry