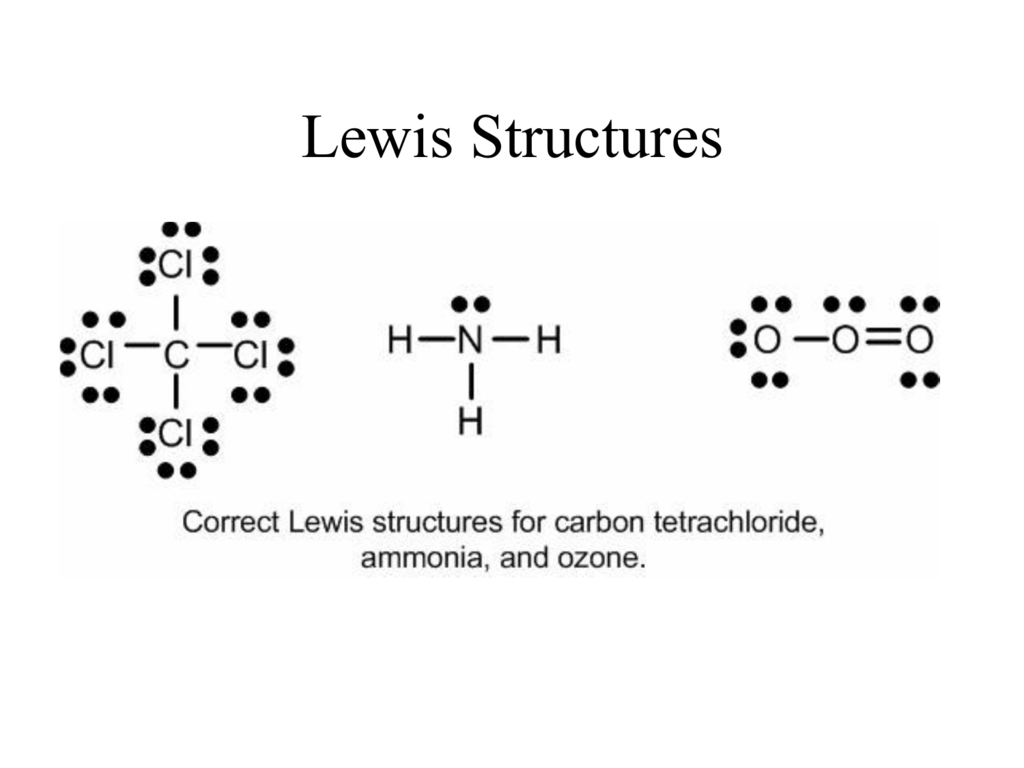
Pcl3 Lewis Structure Resonance
Phosphorus trichloride is a chemical compound having 3 chlorine atoms and 1 Phosphorus atom with one lone pair on Phosphorus atom. The possible structures are called resonance structures and are drawn with a double headed arrow.

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
Please watch the following video on how to draw Lewis structures.

Pcl3 lewis structure resonance. A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure Phosphorus TrichlorideFor the PCl3 structure use the periodic table to find the tot. SOLUTION a The Lewis structure of the SnCl3 ion looks like this. Furthermore a given compound can have several valid Lewis formulas.
Therefore P 6n 2 V 6 5 2 32 0 So there is no double bond. So this molecule is polar. Hree pairs will be used in the chemical bonds between the P and Cl.
By signing up youll get thousands of step-by-step solutions to your homework. Underline The Best If Applicable Electron Geometry Molecular Geometry Polarity Hybridization PCl3 SO3 SO3 2- HCN O3 SeCl4 TeCl4 2 - SO2 Benzene SeF 5 - Propane Ethanol. Therefore the geometry of the electron pairs is tetrahedral with three of the angles occupied by the bonding electron pairs.
PCl3 Valence Electrons. In the Lewis structure for PCl 3 there are a total of 26 valence electrons. Hybridization of PCl3 Phosphorus Trichloride.
Electron dot structures of POCl3. Sncl3 Lewis Structure What is the molecular geometry of sncl3. So PCl3 does not exhibit resonance.
In the PCl 3 Lewis structure Phosphorus P is the least electronegative so it goes in the center. Considering the formula of PCL3. Then learn about resonance and resonance structures for molecules and polyatomic ions.
For example CH 3 CNO can be represented by at least three different but valid Lewis structures called resonance forms or resonance structures shown below. IPCl3 iiCH2Cl2 iiiHCN ivC2H4 vNH3 1In the Lewis structures of _____ the central atom has one lone pair of electrons. Molecule Lewis Structure All Resonance Forms.
Answer to PCl3 ICl3 NO2 IF5 XeF2 IBr4 - SF6 Lewis Structure 3-D Sketch Are There Resonance Structures. Draw the Lewis structure for PCl 3. The lewis structure of PCl3 can be explained as follows.
Sp3 Molecular geometry of PCl3 is trigonal pyramidal with asymmetric charge distribution on central atom. One needs to know the total number of valence electrons for a molecule to construct the Lewis Dot Structure. Afterwards assess your new knowledge with a quiz.
The central atom of Sn is surrounded by a pair of unbound electrons and three single bonds. Draw Lewis structure for these molecules. To draw the lewis structure first of all we need to sum up the valence electrons of all the atoms.
Here Phosphorous 5 valence electrons Chlorine 7 valence electrons 3 Cl 73 21 So total valence electrons 26. However a stable compound such as the above does not exist in multiple states represented by structures I or II or III. Ai only Bi and iv Cii and iv Di and iii Ei and v 2In the resonance form of ozone shown below the formal charge on the central oxygen atom.
To calculate the total number of valence electrons of this molecule we will add up the valence electrons of both Phosphorus and Chlorine atoms. Subtract step 3 number from step 1Use information from step 4 and 5 to draw the lewis structureAlternatively a dot method can be used to draw the lewis structure. For example there are two equivalent Lewis structures for NO 2- as shown below.
Sometimes there are two or more Lewis Structures that can be drawn. Where V 7 5 7 6 7 32 V is the number of valence electrons of the POCl3molecule. So weve used all of our valence electrons all 40.
If you can do those Lewis structures PCl 5 will be easy. OCl2 does not have resonance structures and does not exhibit resonance. Phosphorus has five valence electrons.
PCl 3 is similar to PBr 3 and PF 3. Draw the Lewis Structure for PCl 5. Step 3 4.
Draw a 3-D Sketch c. The resonance Lewis electron dot structures of POCl3 are as follows. The Lewis dot structure.
Now lets move on to the lewis structure of PCl3. PCl3 ICl3 NO2 IF5 XeF2 IBr4- SF6 b. Due to asymmetric shape and difference between the electronegativity the PCl3 molecule is polar in nature.
Get the detailed answer. To Identify The Lewis Structure Resonance Structures Molecular Geometry And Polarity Of Molecules. What type of molecule is PCL3.
Check to make sure that each is a correct Lewis structure. What shape is PCL3. Explain how to draw the Lewis structure for PCl3.
Do they have Resonance Struc.

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube
Is Pcl3 Non Polar Or Polar Why Quora

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

How Is The Electron Dot Structure Of Pcl3 Determined Quora
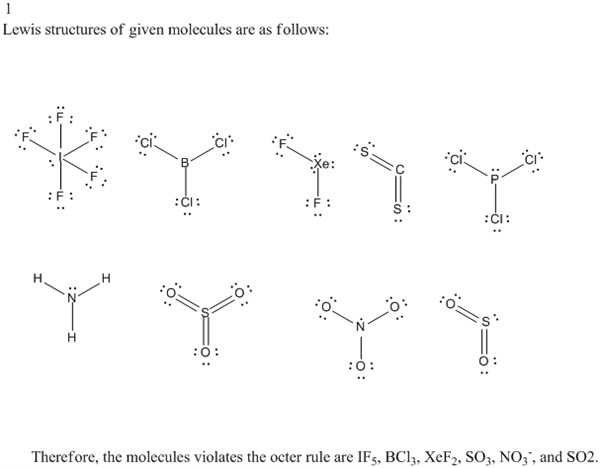
1 Look Again At The Lewis Structures That You Drew Chegg Com

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube
Molecular Geometry Ck 12 Foundation

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
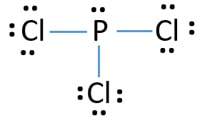
Pcl3 Phosphorus Trichloride Lewis Structure

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Pcl3 Lewis Structure And Molecular Geometry Youtube
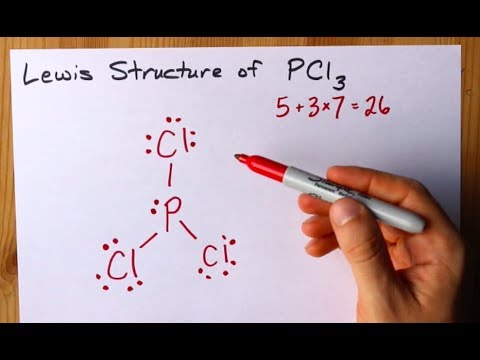
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Molecular Geometry Shape And Bond Angles Youtube