Pf5 Lewis Structure Bond Angle
Molecule F BrNS umber of valence Lewis structure Hybridization 18亡 Number of σ and π electrons n. The angle between the three pairs lying on the central position is 120 degrees and the angle between the axial and equatorial position is 90 degrees.

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules
90 and 120 Molecular Geometry of PF5.
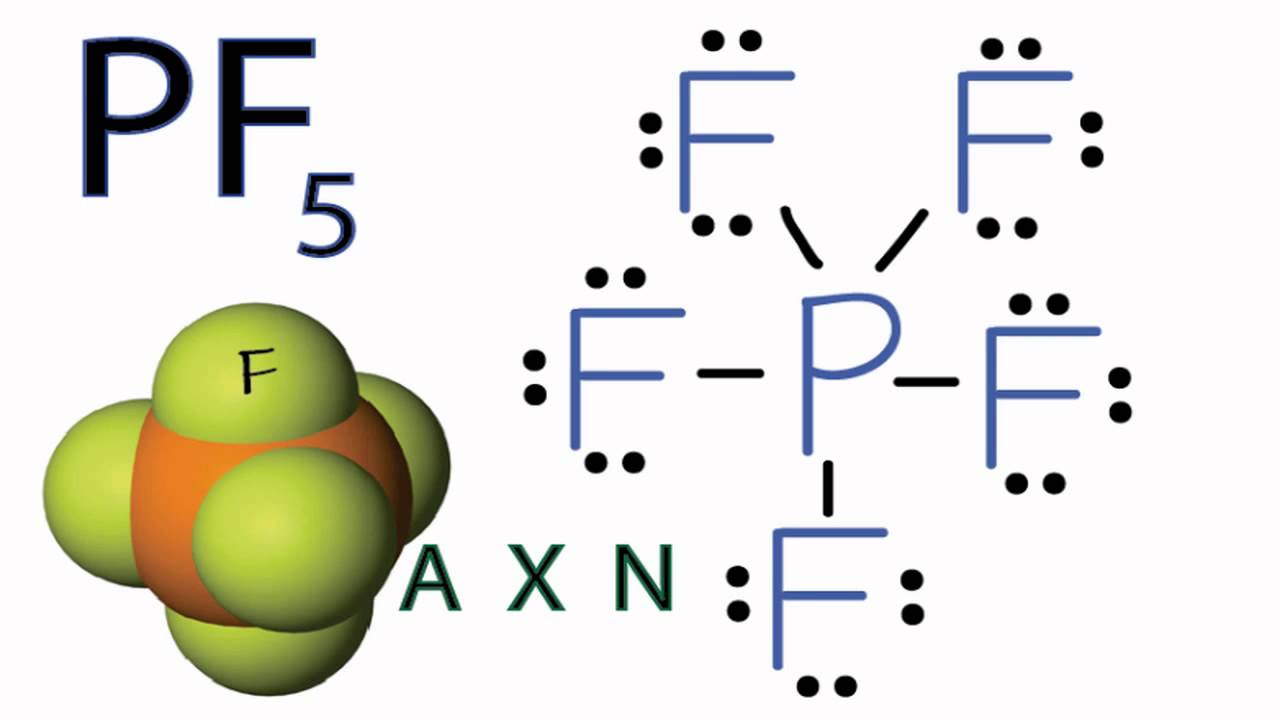
Pf5 lewis structure bond angle. Number of bonded atoms on central atom Molecular shape Number of lone pairs on central atom Bonded-atom lone- pair arrangement BALPA Polarity Central atom steric number Bond angles Bond order N-Br. Examples of molar mass computations. PF5 Bond Angles.
It is used in the semiconductor industry rocket propellant and military applications. Brf5 Lewis Structure Vsepr Can Lewis structures predict the shape of a molecule VSEPR Method by G Dupuis and N Berland The Shapes Of Molecules Predicting the Geometry of Molecules and Polyatomic Ions. This theory explains that the bond angle between the fluorine-phosphorus-fluorine F-P-F is 97.
Bond order is a measure of the stability of the bond between two atoms. Draw a Lewis structure for PF5. I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1.
Phosphorus Pentafluoride PF5 Bond Angles. A quick explanation of the molecular geometry of PF5 including a description of the PF5 bond anglesLooking at the PF5 Lewis structure we can see that there. The angle between the fluorine atoms located in axial and equatorial position is 90 PF5 Shape.
Draw a Lewis structure for PF5. The ligand number is the number of atoms which are bonded to the central atom. As shown in Figure 92.
I3 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram. D Hydro Bromine solution in water. C Hydro Bromine Gas.
B CO has the shortest bond length because of the triple bond. Calculate the longest wavelength of light that has sufficient energy to break the weakest bond in hydrogen peroxide. I2 I- - I3-.
What is the smallest FPF ideal bond angle in the structure. 2 repulsions are minimized by placing the groups in the corners of a tetrahedron with bond angles of 1095. This angle makes the structure bent where the ideal bond angle for the bent trigonal pyramidal structure is 1095.
This anomaly is due to the lone pair of electrons and the smaller size of. 1 bond 3 lone pairs sp3 hybridization. C Hydrogen peroxide is sold commercially as an aqueous solution in brown bottles to protect it from light.
As mentioned earlier the fluorine atoms in PF 5 either occupy the equatorial position or axial one. NaCl CaOH2 K4FeCN6 CuSO45H2O water nitric acid potassium permanganate ethanol fructose. B What is the weakest bond in hydrogen peroxide.
These pairs lie along the equator of the molecule. 5 bonds 0 lone pairs 120 and 90 degree bond angles. The bond angles for the Fluorine atoms in the equatorial position F-P-F is 120.
NF3 PF5 F P F F F F Your Lewis structures should show the sp3 hybridization in NF 3 and the sp 3d hybridization in PF 5The reason NF5 doesnt exist is because N has no d orbitals available for hybridization. What is the smallest FPF ideal bond angle in the structure. This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings.
There are two bond angles for this molecule. There are four electron groups around the central atom. NO2 Molecular Geometry Shape and Bond Angles Note.
The molecular shape is determined by the electron. A Draw the Lewis structure for hydrogen peroxide. PF5 Lewis structure Molecular Geometry Bond angle and.
A -2 -2 -2. The central atom carbon contributes four valence electrons and each hydrogen atom has one valence electron so the full Lewis electron structure is 2. Molecule G Lewis structure Hybridization PFs Number of σ and π bonds Number of.
Rest two pairs lie perpendicular to the equatorial axis known as the axial pairs. In this article we will discuss ClF3 lewis dot structure polar or non-polar its molecular geometry or shape bond angle hybridization etc. It equals the number of bonding electrons in the bond less the number of antibonding electrons divided by two.

Is Sf4 Polar Or Non Polar Sulfur Tetrafluoride In 2021 Math Equations Chemical Formula Molecules
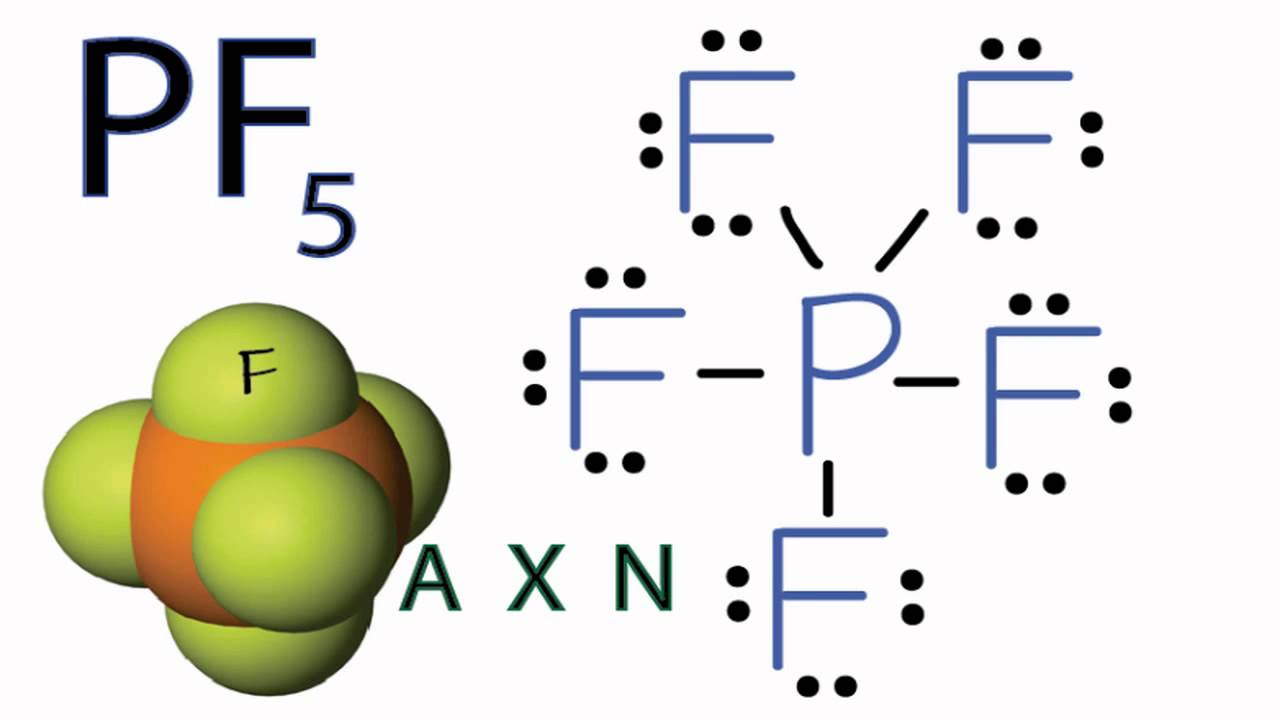
Pf5 Molecular Geometry Shape And Bond Angles Molecular Geometry Geometry Shape Molecular

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Is Co Polar Or Nonpolar Carbon Monoxide In 2021 Carbon Carbon Monoxide Polar

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

P4 Lewis Structure Tetraphosphorus In 2021 Molecules Lewis Electrons

Is Pf5 Polar Or Non Polar Phosphorus Pentafluoride In 2021 Chemical Formula Molecules Phosphorus