What Is The Molecular Geometry Of Pf3
What is the molecular geometry of PF3. The molecular geometry from Exercise 35 is trigonal pyramidal.

Pf3 Molecular Geometry Shape And Bond Angles Youtube
The remaining two non-bonded electrons remain as a lone pair.

What is the molecular geometry of pf3. We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule PF_3 because of its AX_3E status. PF3 has 26 valence electrons. PF3 terahedral trigenal pyramidel.
Enter the molecular geometry of the molecule. Phosphorus has five valence electrons three of which are shared with the three fluorine atoms in PF 3. What molecular geometry does this molecule exhibit.
Enter The Molecular Geometry Of The Molecule. What is the molecular geometry of iodomethane. Bond angle 1095 Because of the lone pair the bond angle will be less than 1095.
Draw a Lewis structure for the molecule. The Lewis structure of PF 3 is. Enter the molecular geometry of the molecule.
Here in this post we described step by step to construct CH3I molecular geometry. Enter the molecular geometry of the molecule. Draw the Lewis structure for PF3 showing all lone pairs 1.
The nitrogen atom and the three carbon atoms at the ends of the molecule make up the trigonal planar center of the molecule. Bond angle 1095. 5 Attempts Remaining Part B What Is The Molecular Geometry Of CO2.
What is the molecular geometry of BeF2. It contains three nonpolar bonds arranged asymmetrically around the central phosphorus atom thus conferring a net dipole moment on the molecule. This gives it a VSEPR notatio.
VESPR stands for valence shell electron pair repulsion. See full answer below. Draw a Lewis structure for the molecule.
Youll find the correct answer below what is the molecular geometry of PF3 The Correct Answer is trigonal pyramidal Reason Explained trigonal pyramidal is correct for what is the molecular geometry of PF3 Answerout. Part A What Is The Molecular Geometry Of PF3. Because of the lone pair the bond angle will be less.
Phosphorus trifluoride or PF3 is a polar molecule. Three single covalent bonds are formed between the phosphorus and fluorine atoms which contributes to the presence of three strong sigma bonds and no pi bonds. PF3 has 26 valence electrons.
View Available Hint s Pyramidal Previous Answers Submit Incorrect. Correspondingly what Vsepr shape is pf3. The valence electrons of Phosphorus are 5 and fluorine has 7 valence electrons in its outermost shell.
The Correct Answer is. The Lewis structure of the tetra-atomic phosphorus trifluoride PF3 molecule shows three fluorine atoms bonded to a single phosphorus central atom. Fill in the below table with the Lewis structure or structures electron domain geometry.
Drawing and predicting the CH3I molecular geometry is very easy by following the given method. IodomethaneCH3I has the composition of one carbon one iodine and three hydrogen atoms. Enter The Molecular Geometry Of The Molecule.
If we talk about the chemical composition of PF3 the molecule consists of one phosphorus atom and three fluorine atoms. Get the detailed answer. Lets count the areas around the phosphorus atom that.
Pf3 molecular geometry Back to Blog. What is the molecular shape of PF3. Enter The Molecular Geometry Of The Molecule.
PF 3 has a trigonal pyramidal molecular geometry. Click to see full answer. PF3 phosphorus trifluoride has one phosphorus central atom surrounded by three fluorine atoms and one lone pair of electrons.
The molecular geometry of Pf3 is trigonal planar. The molecular geometry of the PF3 molecule is _____ and this molecule is _____-trigonal planar polar-trigonal pyramidal polar-tetrahedral unipolar-trigonal pyramidal nonpolar-trigonal planar nonpolar. The molecular mass of PF3 is calculated as Mol mass of PF3 1 30 mol mass of P 3 189 mol mass of F 8796 gmol.
What is the molecular geometry of OF2. Hydrogen iodine and carbon come from the1st 17th and 14th. 1049 a PF3- polar Draw the Lewis structure and determine the molecular geometry.
The VSEPR shape of the molecule PF_3 is trigonal pyrimidal. The tetrahedral molecular geometry of CtiF will have unequal bond vectors so the molecule will have a net dipole. Bond angle 1095 Because of the lone pair the bond angle will be less than 1095.
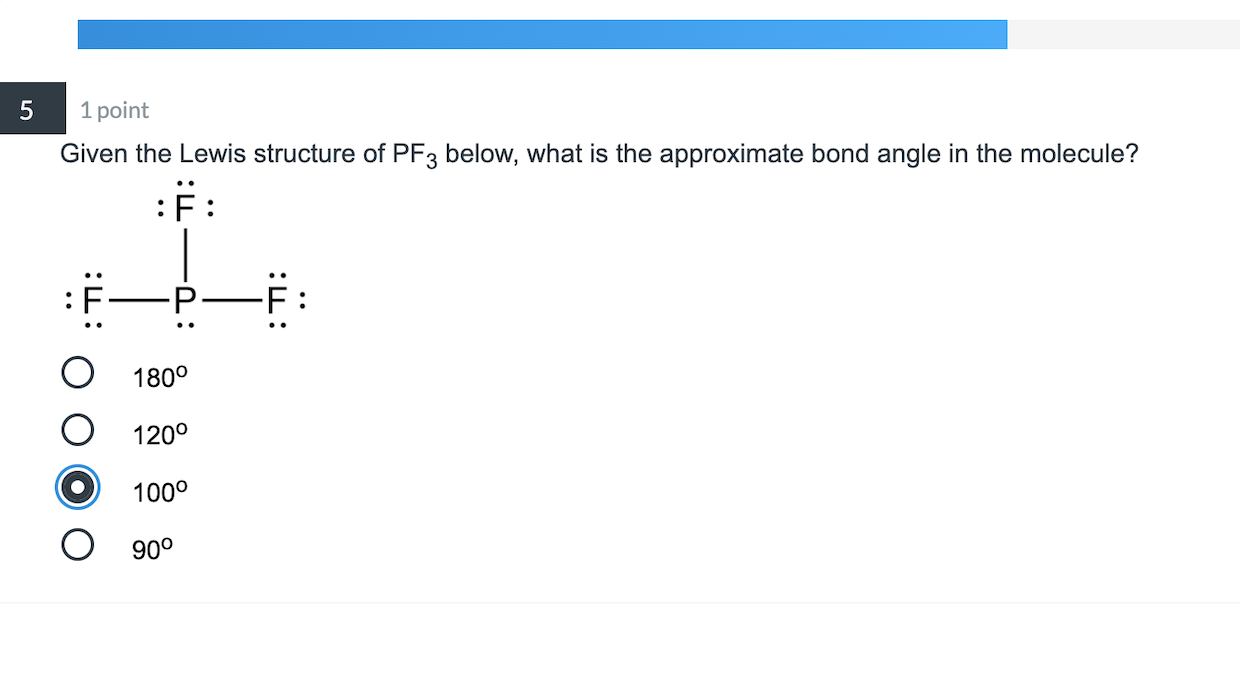
5 1 Point Given The Lewis Structure Of Pf3 Below Chegg Com

Answer The Molecular Geometry Of Pf3 Is B Clutch Prep

Pf3 Draw The Lewis Structure For Pf3 In The Box At Chegg Com
7 1 Point Given The Lewis Structure Of Pf3 Below Chegg Com

What Is The Molecular Geometry Of The Pf3 Molecule
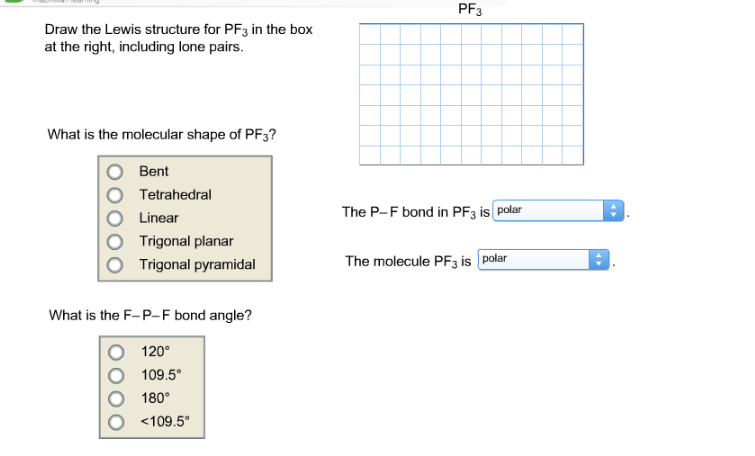
Pf3 Draw The Lewis Structure For Pf3 In The Box At Chegg Com

Pf3 Molecular Geometry Shape And Bond Angles Youtube

Pf3 Molecular Geometry Shape And Bond Angles Youtube

Predict Molecular Geometry Of Pf3 Phosphorus Trifluoride Youtube

Predict Molecular Geometry Of Pf3 Phosphorus Trifluoride Youtube

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

What Is The Molecular Geometry Of Pf3 Study Com

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

A What Is The Molecular Geometry Of Pf3 Clutch Prep
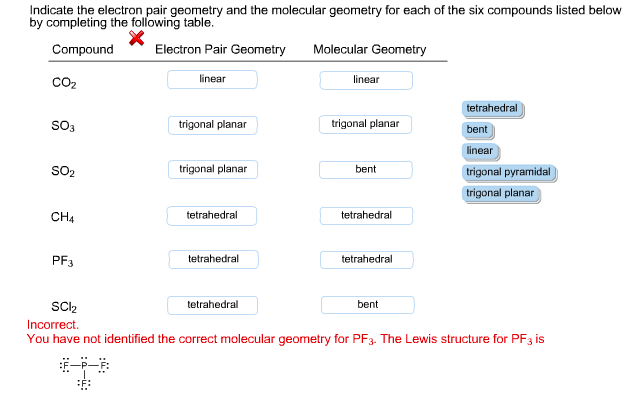
Indicate The Electron Pair Geometry And The Molecular Chegg Com
Pf3 Molecular Geometry Shape And Bond Angles دیدئو Dideo
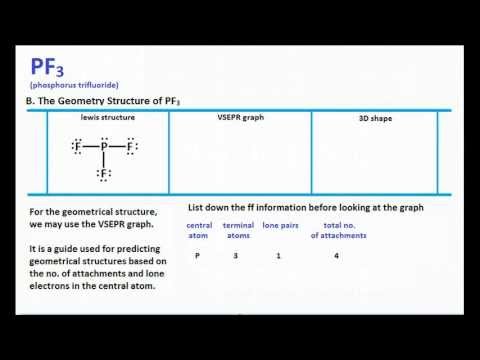
Chemistry Learning Made Easy Pf3 Lewis Structure And Molecular Geometry Youtube

What Is The Electron Geometry Of Pf3 A Bent Angular B T Clutch Prep