Does Xef4 Have Resonance Structures
A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure Xeon TetrafluorideFor the XeF4 structure use the periodic table to find the total n. A molecule can have resonance structures when it has a lone pair or a double bond on the atom next to a double bond.

Xef4 Lewis Structure And Molecular Geometry Youtube
And it has no resonance isomers available.
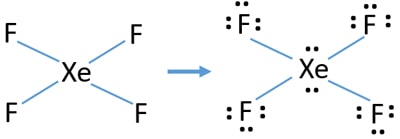
Does xef4 have resonance structures. Answered 3 years ago. Multiple structures are needed to adequately show how electrons are shared. We use dots to represent outer shell electrons and lines to represent the bond type.
Resonance structures are a better depiction of a Lewis dot structure because they clearly show bonding in molecules. As you draw them keep in mind that some of the resonance structures may not satisfy the octet rule. There are three resonance structures SO3 Sulfur trioxide.
Lewis Structure also known as electron dot structure is an essential model of chemical bonding where we use the valence electron concept to schematically sketch a two-dimensional figure of a given molecule. The Lewis model does not account for some behavior of molecules such as O2 being paramagnetic. Try to draw the XeF 4 Lewis structure before watching the video.
Three resonance structures are necessary to account for the fact that the three C-O bonds in the carbonate anion are equivalent and these are shown below. In resonance structure A the pi overlap is very poor due to. For example the NO2 molecule has an odd number of electrons thus the octet rule cannot be satisfied for the nitrogen atom.
No one structure is accurate we have to draw multiple structures. We start with a valid Lewis structure and then follow these general rules. FNO2 resonance structure molecular polarity direction hybrid used.
It is helpful if you. NCCN resonance structure molecular polarity direction hybrid used. The non-equivalent resonance structure contributes differently to the overall structure than the other two.
Each atom has an octet. Nitrous oxide has three inequivalent resonance structures. HOCN resonance structure molecular polarity direction hybrid used.
The resulting structure is an octahedron in which two positions are occupied by lone pairs of electrons. XeF4 resonance structure molecular polarity direction hybrid used. The negative charge on the oxygen atom is conceived to be localized on the oxygen centre.
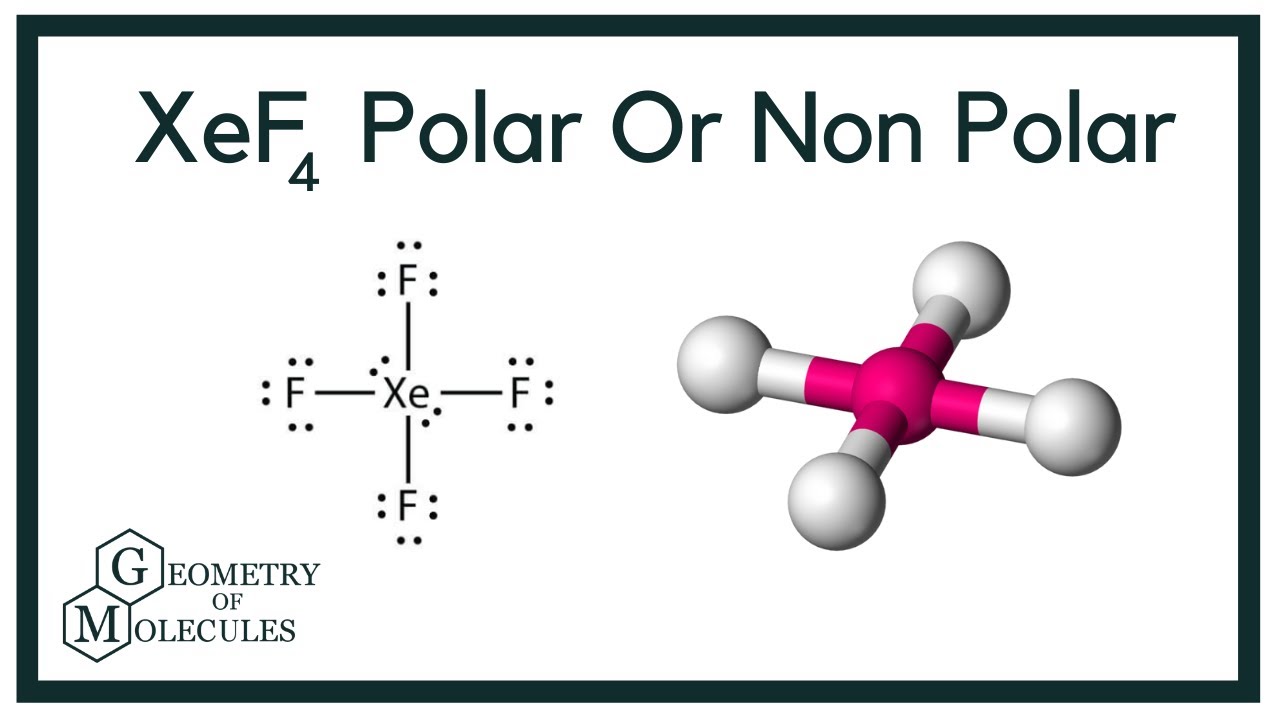
Before deciding how much s-character a hybrid orbital has you must first determine the type of hybrid orbital youre dealing with. So XeF4 is square planner. Xenon is an inert gas element.
IF4 1- resonance structure molecular polarity direction hybrid used. The ceXe-O bonds in ceXeO4 are very weak. The Lewis structure for XeF4 has a.
Lets start with xenon tetrafluoride XeF_4. The better ones have minimal formal charges negative formal charges are the most electronegative atoms and bond is maximized in the structure. Same with resonance structures.
Does CH3OH have resonance structures. Some hints are given. Not all resonance structures are equal there are some that are better than others.
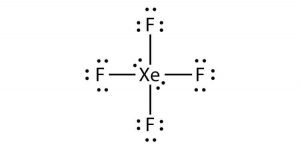
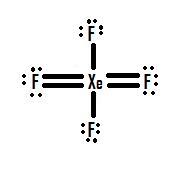
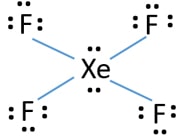
Note that SO3 is a bi. What is the Lewis structure of XeF4. The Lewis structure for XeF4 has a total of 36 valence electrons.
- In the case of sulfur dioxide because of the ability of sulfur to have an expanded octet the third resonance structure is present when sulfur has ten electrons. The double-headed arrow implies a movement of the electrons and an actual shifting of the structure from one to another. Does H3PO4 Have Resonance Structures.
O 3 SO 3 NO 3- CO 3 2-zExample. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
XeF2 Lewis Structure. Remember that Xenon can have more than 8 valence electrons. In XeF4 Xe is in sp3d2 hybridised state.
Draw the Lewis dot structures and resonance structures for the following. While in resonance structure B the xenon has a charge of 4 and each oxygen has a charge of -1. These two structures are called resonance structures and molecules such as benzene which have two or more resonance structures are said to exhibit resonance.
Each atom has an octet in all three resonance structures. With two resonance contributors to account for the equivalence of the two C-O bonds. Orbital hybridization actually tells you s-character.
They are a combination of a peach and plum but you have to use both fruits to describe it accurately. To do that draw the Lewis structures of the molecules. So the resonance structures tell us that the xenon-oxygen bonds in ceXeO4 are some mix of single and double bond character.
In resonance structure A the xenon and oxygens are neutral. This problem has been solved. The molecule has a total of 36 valence electrons - 8 from xenon and 7 from each of the four fluorine atoms.
The simple Lewis structure VSEPR model of bonding is easy to picture but it has limitations. C C0 3 2-. VSEPR theory accounts for the shapes of molecules but does not tell us how those shapes come about.
Does H3PO4 have resonance structures.

How To Calculate The Formal Charges For Xef4 Xenon Tetrafluoride Youtube
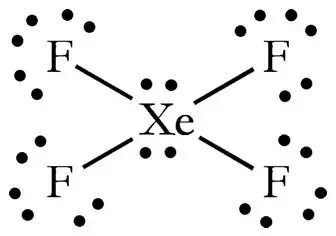
How Many Pairs Of Electrons Are There In The Lewis Structure Of Xef4 Quora

Gen Chem 2 3 Flashcards Quizlet

Xef4 Xenon Tetrafluoride Lewis Structure
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
How Many Pairs Of Electrons Are There In The Lewis Structure Of Xef4 Quora

Why Does The Lewis Structure Of Xef4 Not F Clutch Prep

In The Best Lewis Structure For Xef4 What Is The Formal Charge On The F Atom A 1 B 0 C 1 D 2 Study Com

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
13 12 Exceptions To The Octet Rule Chemistry Libretexts

Xef4 Xenon Tetrafluoride Lewis Structure
Chem 101 Octet Rule Violations

Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
20 How Many Resonance Structures Exist For Ozone O Chegg Com

What Is The Formal Charge On The Xe Atom In Xef4 4 4 2 0 Clutch Prep
Xef4 Xenon Tetrafluoride Lewis Structure

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist