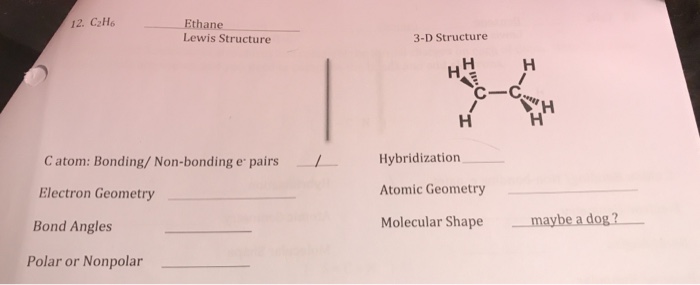
How Many Lone Pairs Does C2h6 Have
Each oxygen makes 1 sigma bond and also needs 2 orbitals for lone pairs of electrons. How many lone pairs are in Hf.
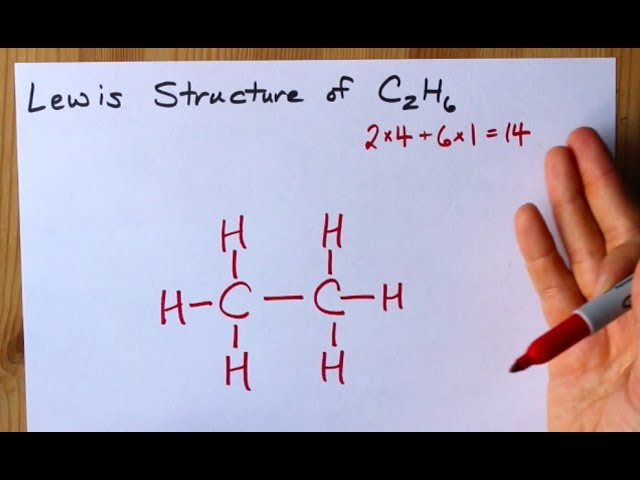
Lewis Structure Of C2h6 Ethane Youtube
With that total electrons around nitrogen atom is going to be ten.
How many lone pairs does c2h6 have. Trigonal bipyramidal electron-pair geometry. The reason water has a bent shape is that the two lone pair of electrons are on the same side of the molecule. Also lone pair present on the carbon is zero.
There is no charge on nitrogen atom too. This is the Lewis Dot structure of C2H6 using up all the fourteen valence electrons. The Lewis dot structure for Cl2 shows how a single covalent bond between the Cl atoms provides a stable octet of electrons around each Cl atom.
It is going to break octal rule because nitrogen atom cannot keep more than eight. 455 20 Views. Click to see full answer.
Therefore hybridization of C2H4 is Sp². Is ch3nh2 a polar molecule. These will again take up a tetrahedral arrangement.
Uncharged carbon has 4 bonds and no lone pairs. Then how many lone pairs are in ICl4. Steps to draw resonance structures for NO 2-You can convert a lone pair of one oxygen atom which already has three lone pairs to make a bond with nitrogen atom.
The arrangement of the electrons and atoms is symmetric in this molecule. This time the bond angle closes slightly more to 104 because of the repulsion of the two lone pairs. How many atoms are contained in 223 mol C2H6.
B the shape of its compound from its Lewis dot structure Determine the number of lone pairs around the Give shape basic shape lone pair bonding pair What has 2 charged clouds 2 bonds and no lone pairs. A step-by-step explanation of how to write the Lewis Dot Structure for C2H6 EthaneFor the C2H6 Lewis structure calculate the total number of valence elect. The positive charge here indicates that it cannot have alone pairs since that would exceed the octet around the nitrogen.
Methyl amine ie CH3NH2 is a. VSEPR CH4 Methane Methane has 4 regions of electron density around the central carbon atom 4 bonds no lone pairs. The Lewis Dot Structure for Cl2.
Micheline Aengen Last Updated. What has 5 charged clouds 2 bonds and 3 lone pairs. Bromine has one bond and three lone pairs and.
According to the ethene lewis dot structure carbon is the central atom and each carbon is attached to three atoms 1 Carbon 2 Hydrogen. Read rest of the answerSimilarly how many lone pairs does chlorine have. This atom will be 2sp hybridized with remaining 2px and 2py atomic orbitals.
Know more about it here. On nitrogen atom there is only a single lone pair. So H 3 0 3 is the hybridization number for C2H4.
From the Lewis structure we can see that the carbon in CO2 must make 2 sigma bonds and it has no lone pairs. Additionally what is the Lewis structure of cl2. This repulsion of the lone pairs of electrons on the oxygen atom causes the bond of the hydrogen to the oxygen to be pushed downward or upward depending on your point of view.
Cl2 is a greenish yellow gas and is a strong oxidizing agent. Would have five electron pairs on the central I atom one of which would be a lone pair. 37 Votes Lewis structure of CH2Br.
Click to read more on it. All the valence electrons are used up to make the molecules stable structure hence it doesnt have any lone pairs or nonbonding pairs of electrons. While exceeding the octet is not impossible in fact the octet rule applies only to the elements in the second row it is still very important in organic chemistry because carbon and the other elements in the second row do obey the.
Following the same logic as before you will find that the oxygen has four pairs of electrons two of which are lone pairs. Lone pairs on that central atom. In this manner how many lone pairs does ch5n have.
Beside above why does h2o have two lone pairs. H with two lone pairs on the O giving 2 bonding pairs and 2 non-bonding pairs lone pairs. What has 6 charged clouds 4 bonds and 2 lone pairs.
Four bonds and a lone pair that is a total of 10 electrons. The shape is not described as tetrahedral because we only see the oxygen and the hydrogens - not the lone pairs.

C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist

C2h6 Molecular Geometry Shape And Bond Angles Youtube

C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist

C2h6 Ethanelewis Structure Clutch Prep

Is C2h6 Polar Or Non Polar Ethane Youtube

C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Ethane Lewis Structure 12 C2h6 3 D Structure C C C Chegg Com

C2h6 Ethanelewis Structure Clutch Prep
Lewis Structure Of C2h6 Biochemhelp
Observation 1 Geometries Of Molecules Chemistry Libretexts

C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Basic Molecular Modelling The Three Dimensional Arrangement Of Atoms
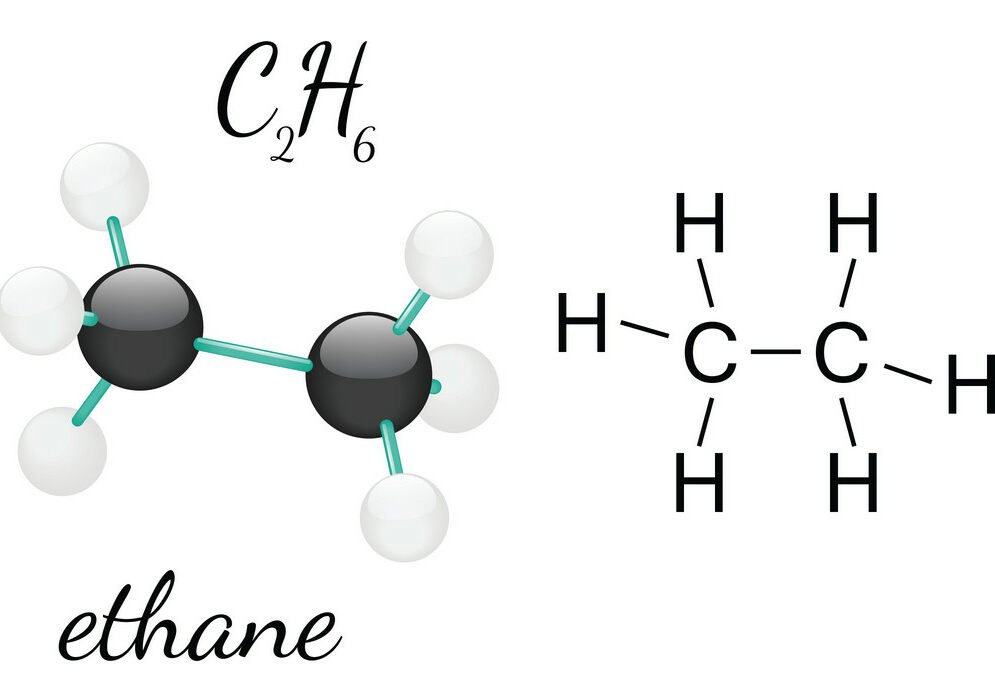
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape
What Is The Molecular Geometry Of C2h6 Quora
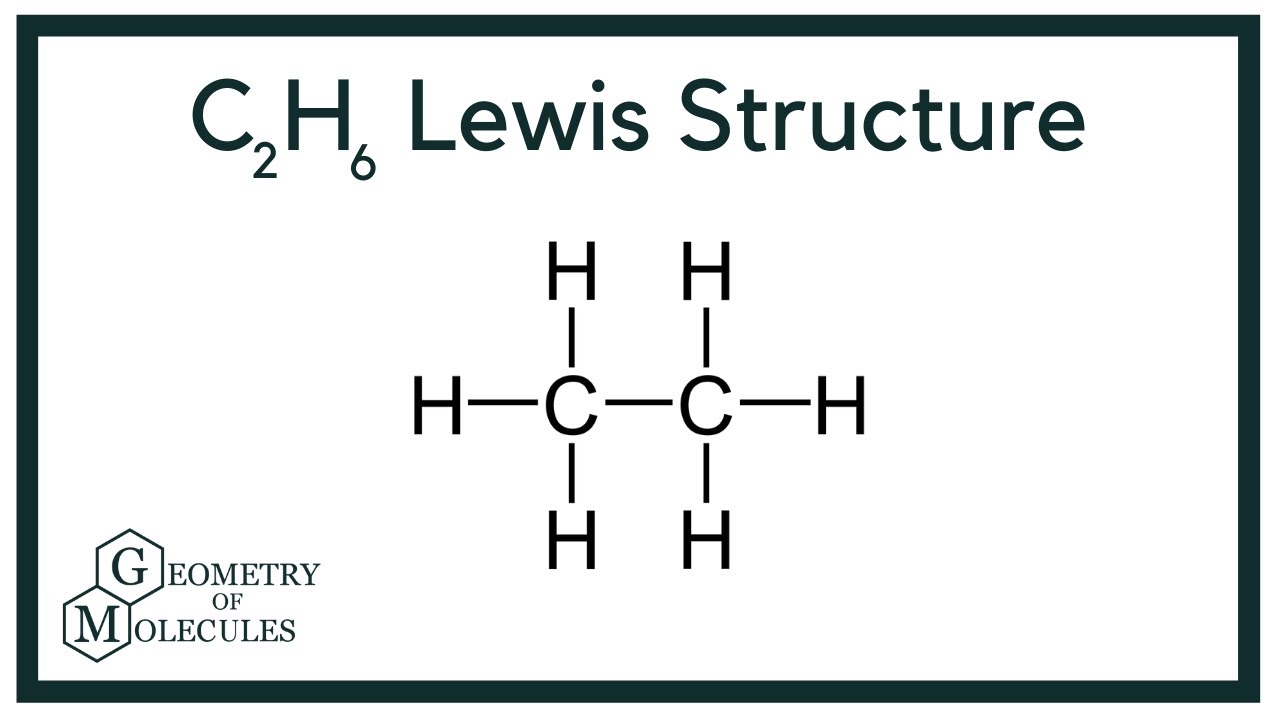
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape
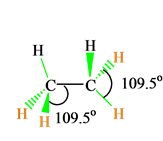
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape

C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape
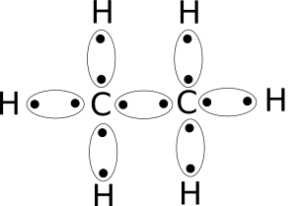
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape