Best Lewis Structure For Co2
Carbon is the least electronegative that means it stays at the center. These properties in addition to its small state makes it so that carbon dioxide has a low melting point and is mostly in the gaseous phase at STP Standard Temperature and Pressure.

Co2 Lewis Structure Carbon Dioxide Youtube
Draw Lewis Structure For Co2 co2 lewis structure dot dioxide carbon electron diagram electrons resonance pairs structures bonds many covalent lone valence oxygen ionic double dioxide lewis carbon co2 structure molecular dot draw structures chemical chemistry monoxide lewis co2 structure carbon dioxide draw centre oxygens either put going then side co2 dot lewis diagram structure.

Best lewis structure for co2. Posted another one can you check that one too. The Lewis dot structure is drawn with letters that represent the atoms of the element and then a number of dots or dashes surrounding these letters. GCSE-Covalent Bonding-Electron Dot Structure of CO2 - YouTube.
Draw The Lewis Structure For The Nitrosyl Chloride. Answered Oct 17 2020 by RobinHood. The carbon dioxide chemical formula is CO2.
One of these oxygen atoms takes a proton Ion XH and forms a group -OH. There are two double bonds around carbon atom in the CO 2. Out of these the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website.
Answered Sep 16 2016 by Nutellamaniac. Lewis dot structure of CO2. The lewis dot structure of CO2 gives it some unique properties.
For the CO Lewis structure youll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. The best Lewis structure is one in which has the fewest formal charges. Answered Oct 17 2020 by lizhover.
How To Read A Lewis Dot Structure. Therefore it is nonpolar and relatively unreactive. A -1 B 0 C 1 D 2.
A 2 B -1 C 1 D 0. Draw the Lewis structure. More questions like this In the best Lewis structure for.
Calculate the formal charge on each atom. Answered Sep 16 2016 by Berger77. Thus this structure has a better chance of being a lewis structure of CO32-ions.
Please help me in solving this I will pay. Here in this post we described step by. Classify the bond in CO2 as polar covalent non polar covalent or ionic.
The Lewis structure for CO has 10 valence electrons. Asked Sep 16 2016 in Chemistry by PSG10. We offer a huge of best lewis structure for co2 news and articles here.
Lewis dot structure of CO2. Carbon dioxide CO 2 lewis structure has two oxygen atoms and one carbon atom. CO2 Lewis structure So CO2 4 62 16.
The first structure has no formal charges so the best Lewis structure for. What Is Co2 Lewis Structure And How To Draw It. Lewis co32- carbonate ion questions Ask your chemistry questions and find answers In carbonate ion there are two oxygen atoms that have a -1 charge on each one.
D 0 0 votes. Lets go over the Lewis structure and find out how to interpret this representation of carbon dioxide. Since there are no lone pairs on the atom it is a linear structure which makes the charges cancel it.
So total valence electrons are 16. Asked Oct 17 2020 in Chemistry by kxtheryn. CO 2 Carbon dioxide Lewis Structure and Shape.
I also go over hybridization shape and bond angles. Co2 lewis structure polar or nonpolar. As before add valence electrons to give each atom an octet.
No lone pairs on carbon atom and each oxygen atom has two. Dots can be used to represent the shared electrons within the bonds of the atoms but dashes can be used to. Co2 lewis structure polar or nonpolar.
Drawing CO2 Lewis Structure is very easy to by using the following method. I am stuck on my homework and missing deadline. In the best Lewis structure for CO2 what is the formal charge on the C atom.
I quickly take you through how to draw the Lewis Structure of CO2 Carbon DiOxide. Answered Oct 17 2020 by Schmubby. CO2 Lewis Structure - How to Draw the Dot Structure for.
2 Day Business Management Masters. In order to complete the octets for all of the atoms in the structure. After determining how many valence electrons there are in CO place them around the central atom to complete the octets.
This website uses cookies to improve your experience while you navigate through the website. There are three possibilities. - You Ask.
Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structureThe Lewis structure for CO2 has a total of 16 valence electrons. In the best Lewis structure for CO2 what is the formal charge on the C atom.

Co2 Lewis Structure Molecular Geometry Molar Mass Hybridization
Co2 Lewis Structure Molecular Geometry And Hybridization

Co2 Lewis Structure Easy Hard Science

Co2 Lewis Structure Easy Hard Science
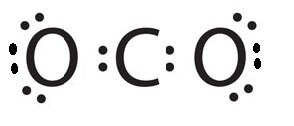
Co2 Lewis Structure Molecular Geometry And Hybridization

Resonance Structures For Co2 Carbon Dioxide Youtube

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube
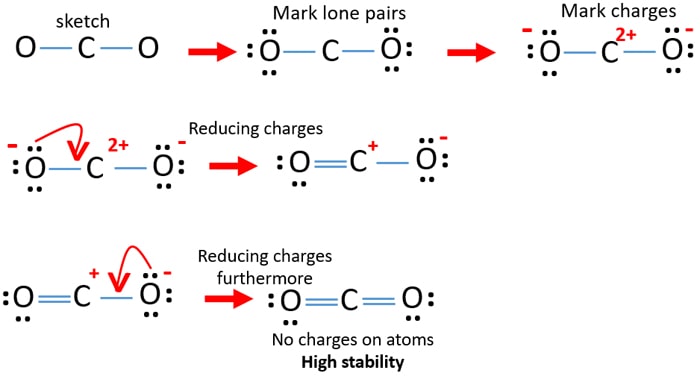
Co2 Carbon Dioxide Lewis Structure And Shape

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
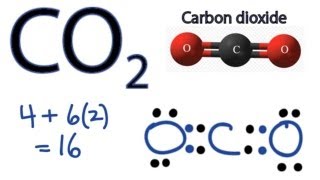
Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Makethebrainhappy The Lewis Dot Structure For Co2

Lewis Electron Dot Structures Detailed Explanation With Examples Videos

Co2 Lewis Structure Molecular Geometry And Hybridization
![]()
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2

Makethebrainhappy The Lewis Dot Structure For Co2

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube
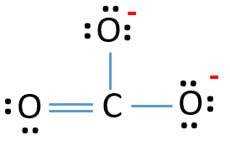
Lewis Structure For Co32 Carbonate Ion
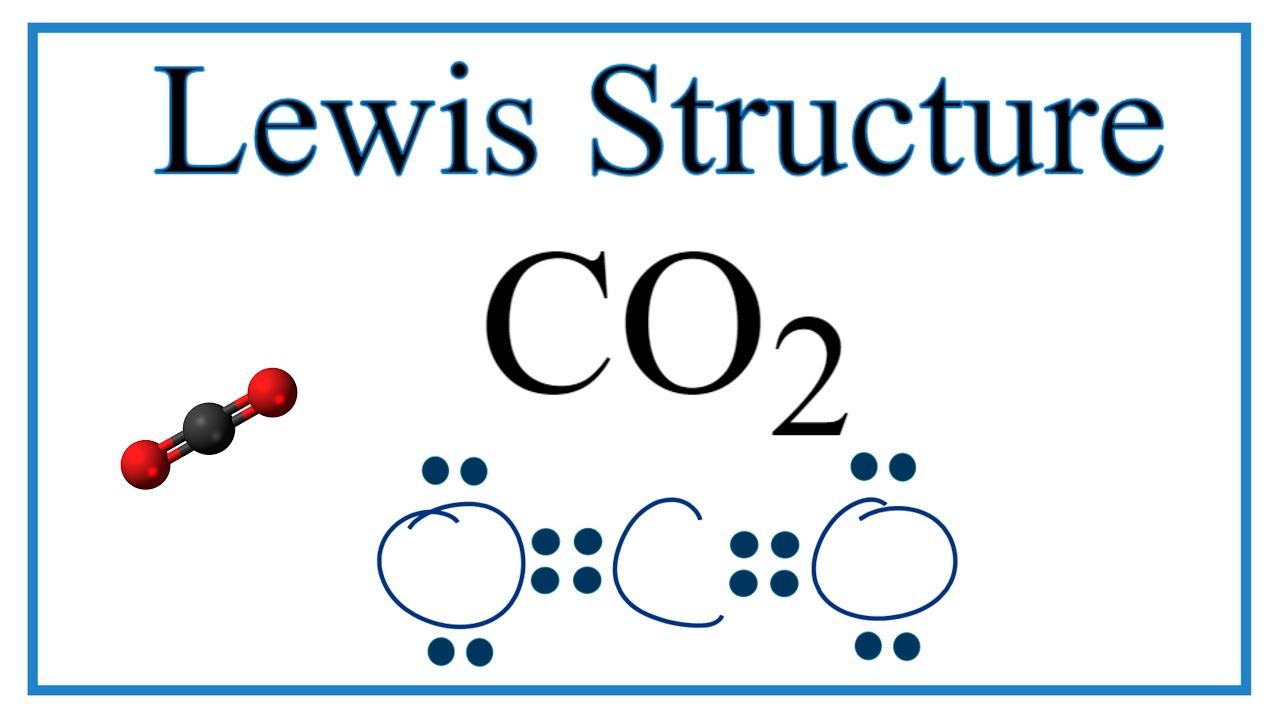
Resonance Structures For Co2 Carbon Dioxide Youtube