Brf5 Lewis Structure Lone Pairs
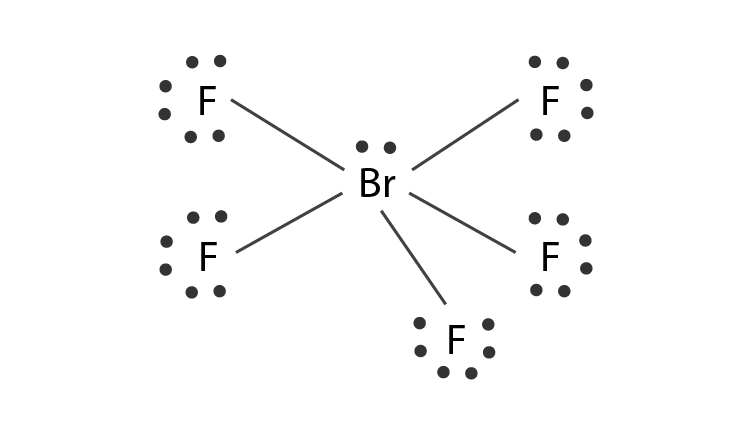
The polarity is best found by first drawing the Lewis dot structure for BrF5. 5 of them form Sigma covalent bonds with 5F atoms.

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
In IF5 considering I to be the central atom it has 7 valence electrons.
Brf5 lewis structure lone pairs. Draw the Lewis Structure of BrF5 and determine the number of lone pairs around the central atom. Put Bromine in the center and five fluorine atoms on the sides. Being in period 4 of the periodic table it is capable of having this expanded octet.
Five valence electrons of bromine will be used to form sigma bonds with 5 F atoms. The are no lone pairs around the central atom. Lone pairs are found in one of the hybrid orbitals.
If the molecule has an AX5N1 generic formula the molecular geometry will be square pyramidal and the electron geometry will be octahedral according to the VSEPR theory. We know bromine is the center atom with 5 bound Br-F pairs of electrons and one lone pair. 5 F atoms and 1 lone pair.
The steric number is an important term here which we need to find out for any VSEPR calculation. Atoms and lone pairs are treated differently. Hope this article helps you all.
There is one bromine atom and. The central atom bromine forms 5 sigma bonds with fluorine atoms. Experts are tested by Chegg as specialists in their subject area.
The structure of BrF5 could have been an octahedron but because of the presence of a lone pair of electrons the structure rearranges itself into a square pyramidal shape. Bromine pentafluoride is an interhalogen compound. Draw a valid Lewis structure showing all nonbonding electrons for dichloromethane CH2Cl2.
A step-by-step explanation of how to draw the PF5 Lewis Dot Structure Phosphorus PentafluorideFor the PF5 structure use the periodic table to find the tot. Br is the central atom. There for this forms a Pyramidal structure with a lone pair.
The electron-domain geometry of BrF5 is AX6 or octahedral. We are now left with the final Lewis structure of BrF 5. We have three Fluorine atoms surrounding the central Br atom therefore three bond pairs.
E Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 5 E 1. There are six electron groups around the central atom. There are 5 atoms and 1 lone pair around the central atom.
In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. The two remaining valence electrons act as a lone pair. The lone pairs are around iodine and sigma bonds formed between the F and Br.
We have two lone pairs on the Bromine atom an exception to the octet rule. See the answer See the answer done loading. Draw the Lewis structure of acetic acid CH3CO2H clearly.
Because of the BrF5 Lewis structure. The electron geometry of BrF5 in its Lewis structure is octahedral and the hybridization is sp3d2. Let us have a look at the Lewis Structure again.
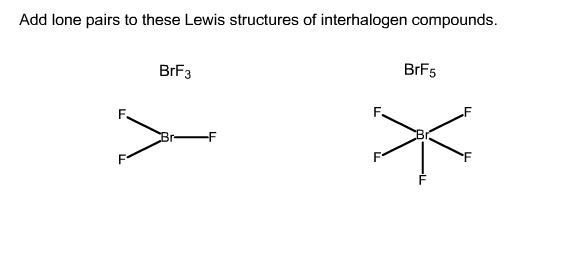
Add lone pairs to these Lewis structures of interhalogen compounds. It is observed here that the central atom Bromine has 12 valence electrons attached to it. Important Points To Remember.
The molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution. Alternatively a dot method can be used to draw the lewis structure of BF 3. The molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution.
BrF5 has the general molecular geometry formula AX5N1. With the help of the BrF5 lewis structure we know bromine is the central atom that has 5 bonded pairs of electrons and one lone pair. The molecule will consist of one lone pair.
So according to the VSEPR chart if the molecule has an AX 5 N 1 generic formula then the molecular geometry for that molecule will be square pyramidal and electron geometry is octahedral. According to the VSEPR theory the lone pair of electrons exerts the greatest repelling effect as they are closer to the central atom in comparison to the bonding pair of electrons. The molecule has a central bromine atom that is surrounded by five fluorides and a lone pair of electrons.
We review their content and use your feedback to keep the quality high. The two extra lone pair electrons go on the Br. BrF5 or bromine pentafluoride is a polar molecule.
Calculate the total valence electrons in BF 3 molecule. The generic formula for BrF5 is AX 5 N 1. The hybridization of BrF5 is determined by the number of sigma bonds and lone pairs.
Put a pair of electrons connecting the side atom with central atomPur remaining electrons on the side atomsMake sure each side atom get 8 electrons to get. And 2 electrons form a lone pair of electrons. Electron groups include lone pairs and atoms.
The molecule has a central bromine atom that is surrounded by five fluorides and a lone pair of electrons. Provide a valid Lewis structure for a molecule with the molecular formula CH2O2. The lewis structure of Bromine pentafluoride is a bit complicated as it has an exceptional case like expanded octet.
This lone pair is then attached to the central Bromine atom. In BrF 5 one 4s three 4p and two 4d orbitals take part in hybridization. Lewis dot structure of BrF 5.
Who are the experts. There are seven valence electrons in the central atom iodine and 5 electrons form 5 sigma bonds with the atoms F. BrF5 or bromine pentafluoride is a polar molecule.

Make A Sketch Of Brf5 Clutch Prep
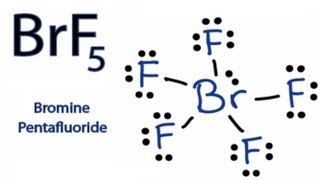
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube
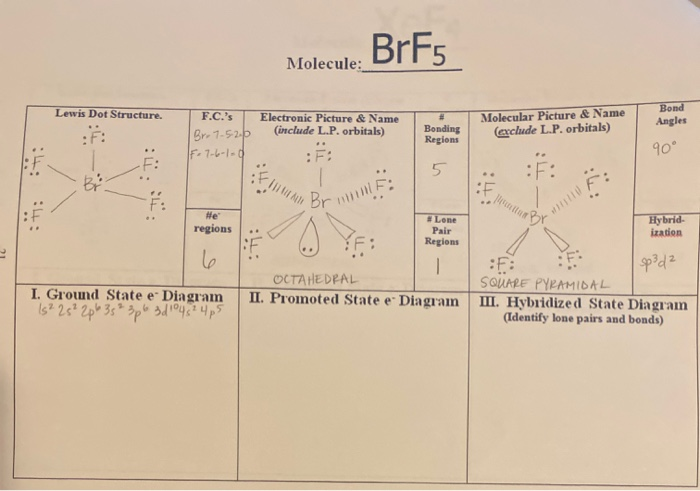
Brf5 El Brillill Molecule Lewis Dot Structure Chegg Com

Would The Lone Pair Be In The Equatorial Plane Or The Axial Plane For Bromine Pentafluoride Chemistry Stack Exchange

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
Lewis Structure Of Brf5 Biochemhelp

Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube

Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube

Bond Angles In Brf5 Chemistry Stack Exchange

Add Lone Pairs To These Lewis Structures Of Chegg Com
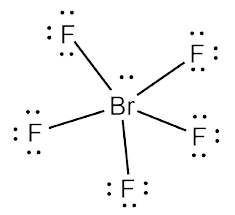
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

The Central Atom In Brf5 Has How Many Bonding Pairs Of Electrons And How Many Non Bonding Pairs Of Electrons Study Com
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Bromine Pentafluoride Brf5 Is Sometimes Clutch Prep
What Type Of Hybridized Orbital Is Used By The Central Atom Of Brf5 Quora