Brf5 Lewis Structure Molecular Geometry
What is the Lewis dot structure of CCl4. The Lewis structure of the BrF5 molecule gives information about the bond pairing and lone pair of the molecule.

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
The molecular geometry of AlCl3 is trigonal planar with each Al-Cl bond angeled 120 to each other and its electron geometry is also trigonal planar.

Brf5 lewis structure molecular geometry. An explanation of the molecular geometry for the BrF5 ion Bromine pentafluoride including a description of the BrF5 bond angles. From the Lewis dot structure of BrF5 it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 296 and 398. In the AlCl3 lewis structure a total of 9 lone pairs are present but no lone pair on the central atom.
It tells us about the nature of the bonds present while also giving insight into the molecular geometry and bond angles of the compound. Once we know how many valence electrons there are in BrF5 we can distribute them around the central atom with the goal of filling the outer shells of each atom. The carbon atom is the middle element in CH3I molecular geometry with four electrons in its outermost valence electron shell whereas the.
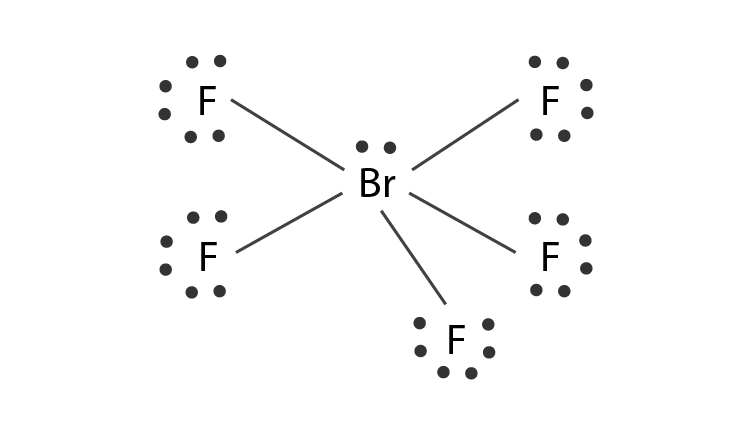
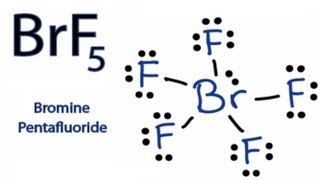
Bromine pentafluorideBrF5 electron geometry is. Draw the Lewis structure for the molecule. A step-by-step explanation of how to draw the BrF5 Lewis Dot Structure Bromine pentafluorideFor the BrF5 structure use the periodic table to find the tota.
To do so we first need to draw a Lewis structure for BrF 5. BrF 5 Molecular Geometry And Bond Angles BrF 5 molecular geometry is said to be square pyramidal with a bond angle of 90 o each. In the carbon tetrachloride molecule four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds.
The possible electron pair and molecular geometries are. Lewis Structure is the diagrammatic form given to the skeleton of any molecular composition or ion formed with the help of the constituent elements the valence electron concept and the bond. Based on VSEPR theory predict the electron-pair and molecular geometries for this molecule.
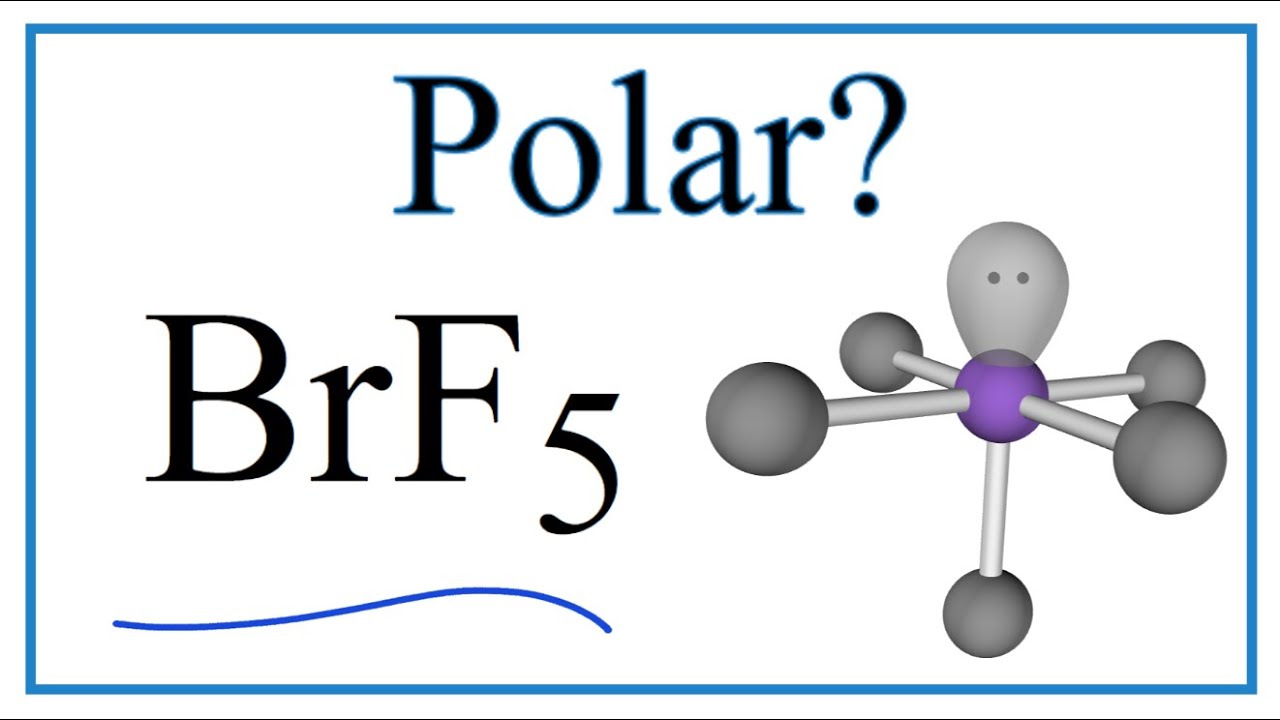
The molecular geometry of BrF5 is square pyramidal and its electron geometry is octahedral. BrF5 or bromine pentafluoride is a polar molecule. SOLUTION a The Lewis structure for the SnCl3-.
Drawing the Lewis Structure for BrF5 For the BrF5 Lewis structure the total number of valence electrons found on the periodic table is 42. Drawing the Lewis Structure for BrF 5 Video. The outermost valence electrons of the CH3I molecule must be understood while considering the Lewis structure of the molecule.
For this we need to do the following steps. Lewis Dot of Carbon TetraChloride CCl4 TetraChloromethane. BrF5 Lewis Structure.
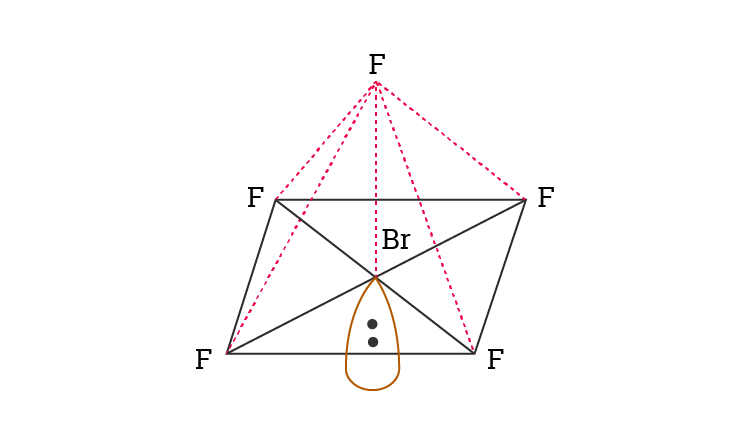
What is the molecular geometry of NCl3. As shown above due to its asymmetrical square pyramidal structure and the presence of a lone pair BrF 5 is considered a polar molecule. The molecule has a central bromine atom surrounded by five fluorides and a pair of electrons.
CBr4 is a nonpolar molecule because of the zero net dipole moment caused by its symmetrical structure. The central Sn atom is surrounded by one nonbonding electron pair and three single bonds. Bend V shape Fig 1.
The electron and molecular geometry of CBr4 are tetrahedral as per VSEPR theory. The hybridization of CBr4 is Sp 3 and the bond angle of 1095. The difference in electronegativity between the Bromine and Fluorine atoms also contributes to unequal charge distribution on the central Bromine atom.
C is the Best Lewis structure for OCl 2 with 1109 o bond angle. Therefore this proves that BrF 5 is a polar molecule. In the CBr4 lewis structure a total of 12 unshared and 4 shared pairs are present.
When a molecule is formed it consists of several atomic elements either the same or different that come together and form single or multiple bonds to form the above-mentioned molecular structure. The Molecular geometry of NCl 3 is Trigonal pyramidal Fig1. Bromine pentafluorideBrF5 is made by a central bromine atom with surrounding five fluorine atoms in square pyramidal geometry.
The Lewis structure of a compound represents a schematic arrangement of all the atoms present in the compound. The total valence electron available for the BrF5 lewis structure is 42. The outermost electrons are also shown attached to their constituent atoms as well.
The difference between both the values is 102 which is greater than 04 so the BrF5 molecule is a polar molecule. The hybridization of BrF5 is Sp³d². Place the following in order of decreasing X-A-X bond angle where A represents the central atom and X represents the outer atoms in each molecule.
Check out the below picture to clarify your confusion further. Bromine pentafluoride is polar in nature. The Lewis structure of BrF5 is shown below.
Bromine PentafluorideBrF5 is a polar molecule because the molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution and with a bong angle of 90. Read More About Hybridization of Other Chemical Compounds Hybridization Of XeF4. Because the molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution and with a bong angle of 90.
Determine the central atom in this molecule. Calculate the total number of valence electrons present. The electron geometry for.
Because of this symmetrical geometry CCl 4 is non-polar.

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5

Explain The Shape Of Brf5 Brainly In
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Bond Angles In Brf5 Chemistry Stack Exchange

Brf5 Lewis And 3 D Structure Dr Sundin Uw Platteville
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
Is Brf5 Polar Or Nonpolar Bromine Pentafluroide Youtube

What Is The Molecular Geometry For Brf5 A Clutch Prep

Lewis Structure For Brf5 Molecular Geometry Bond Angle Hybridization Polar Or Nonpolar Quizalize

Would The Lone Pair Be In The Equatorial Plane Or The Axial Plane For Bromine Pentafluoride Chemistry Stack Exchange

Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis And 3 D Structure Dr Sundin Uw Platteville

Bromine Pentafluoride Brf5 Is Sometimes Clutch Prep

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle