Lewis Dot Structure Of Hno3 Class 11
Lewis dot structures can be written by adopting the following steps. Write electron dot structure of ethane of molecule C2H6 brainlyinquestion2073273.
Oxygen contains 6 valence electrons which form 2 lone pairs.

Lewis dot structure of hno3 class 11. Reason In Lewis representation the least electronegative atom occupies the central position in the moleculeion. Subtract 1 e for each charge Spot the central and terminal atoms and write the skeletal structure. Ask Questions for CBSE Class 11 Chemistry Chemical.
Draw the molecular skeleton draw a single bond from each surrounding atom to the central atom subtract 2 valence electrons for each bond. HNO 3 Nitric acid Lewis Structure. Since it is bonded to only one carbon atom it must form a double bond.
In 1916 Kössel and Lewis developed an important theory of chemical combination between atoms known as the electronic theory of chemical bonding. Octet rule deals with the stability of an atom. Write Lewis structure of the following compounds and show formal charge on each atom.
12 Lewis Structure Of Hno3. Carbonate ion is a common polyatomic ion found in limestone baking powder and baking soda. Nh4 lewis structure dot diagram ammonia nh4cl nh3 electron nh co2 ammonium structures chemistry drawing represents following which created.
Let us consider the electron dot structure of sulphuric acid The chemical formula of Sulpuric acid is H 2 SO 4. Ammonia NH3 Lewis Dot Structure YouTube. Lewis structure of carbonic acid H2CO3.
Accordingly atoms can combine either by transferring valence electrons from one atom to another gaining or losing or by sharing valence electrons so as to have one octet in their valence shells. In the previous video we saw some steps for drawing dot structures in this video were going to use those same steps to draw a few more dot structures but were also going to talk about how formal charge relates to dot structures so well get back to this definition in a minute for right now lets draw a quick dot structure for the ammonium cation so NH 4 plus the first thing you do is find. A step-by-step explanation of how to draw the O3 Lewis Dot Structure OzoneFor the O3 structure use the periodic table to find the total number of valence.
Lewis Dot Structure. It does not matter which atom they come from. Sulphur atom 2 8 6 is the central atom have six valence electrons as it has vacant d-orbital sulphur can show variable valencies.
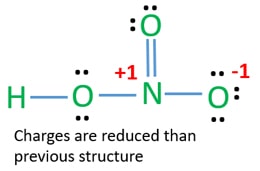
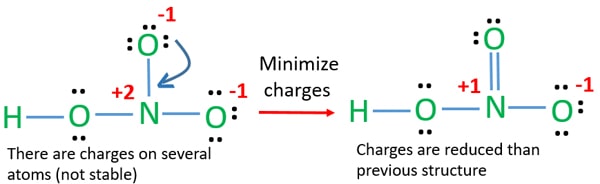
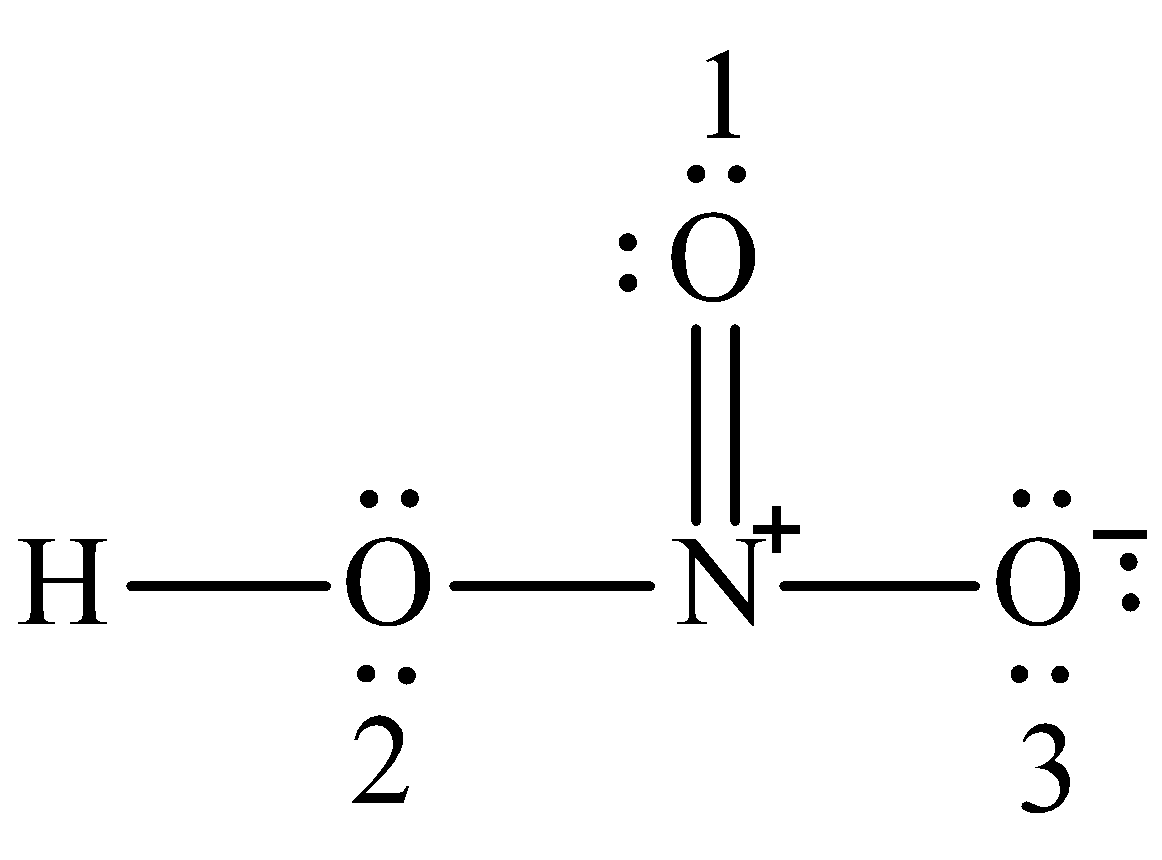
The large bracketed version is the resultant delocalised charge around the whole. The Lewis electron dot structures of a few molecules are illustrated in this subsection. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom.
Resonance Structures of Carbonate CO 32 Ion. Count all valence electrons. Assign formal charges to atoms in the structure.
It exists only in the form of its salts carbonates acid salts hydrogen carbonates amines carbamic acid and acid chlorides carbonyl chloride. Lewis Structure Examples. P 6n 2.
In the Lewis structures of N F 3 and C O 3 2 nitrogen and carbon occupy the central position whereas fluorine and oxygen occupy the terminal positions. HNO3 NO2 H2SO4 asked Aug 22 2018 in Chemistry by Sagarmatha 545k points. Books How To Draw Lewis Dot Structure For Hno3 U Can.
Choose a center atom. The other case of stability rule is. The central atom of this molecule is carbon.
Addition of acid to the carbonate ion causes the formation of carbonic acid which decomposes rapidly into water and carbon dioxide. Be the first to rate this page. Limitations Of Octet Rule.
Moore 2015-08-10 Now you can score higher in chemistry Every high school requires a course in chemistry for graduation and many universities require the course for majors in medicine engineering biology and various other sciences. Calculate the number of electrons which must be shared through π bonds pi bonding P in the molecule. If we draw it like the one on the right for hno3 in order to satisfy the octet rule the nitrogen atom would form 1 double bond and 2 single bonds.
The Lewis Dot Structure for CO 3. Add 1 e for each charge. Lewis Structure of CO2.
Draw Lewis structures step by step. According to this rule an atom is stable if its outermost shell have total of 8 e. In baking the carbon dioxide that is released causes bread to rise and.
It is a special case of the stability rule of atoms which suggests that the outermost shell should be completely filled for an atom to be stable. Sum of valence electrons from all atoms. Chemistry I For Dummies-John T.
HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element. An animation for nitric acid hno3 structure with lewis structure on the left side.

What Is Nitric Acid Structure Uses Formula Video Lesson Transcript Study Com

The Lewis Structure Of Hno3 Chemistry Stack Exchange
How Is The Lewis Dot Structure For Nitric Acid Determined Quora
Hno3 Nitric Acid Lewis Structure

Write Lewis Structure Of The Hno3 And Show Formal Charge On Each Atom

How Is The Lewis Dot Structure For Nitric Acid Determined Quora
Hno3 Nitric Acid Lewis Structure

Give The Lewis Dot Structure Of Hno3 Brainly In
Write Lewis Structure Of The Hno3 And Show Formal Charge Class 12 Chemistry Cbse
Write Lewis Structure Of The Following Compounds And Show Formal Charge On Each Atom Hno3 No2 H2so4 Sarthaks Econnect Largest Online Education Community

Lewis Dot Structure Of Hno3 How To Draw Lewis Structures Class 11 Chemistry Chemical Bonding Youtube
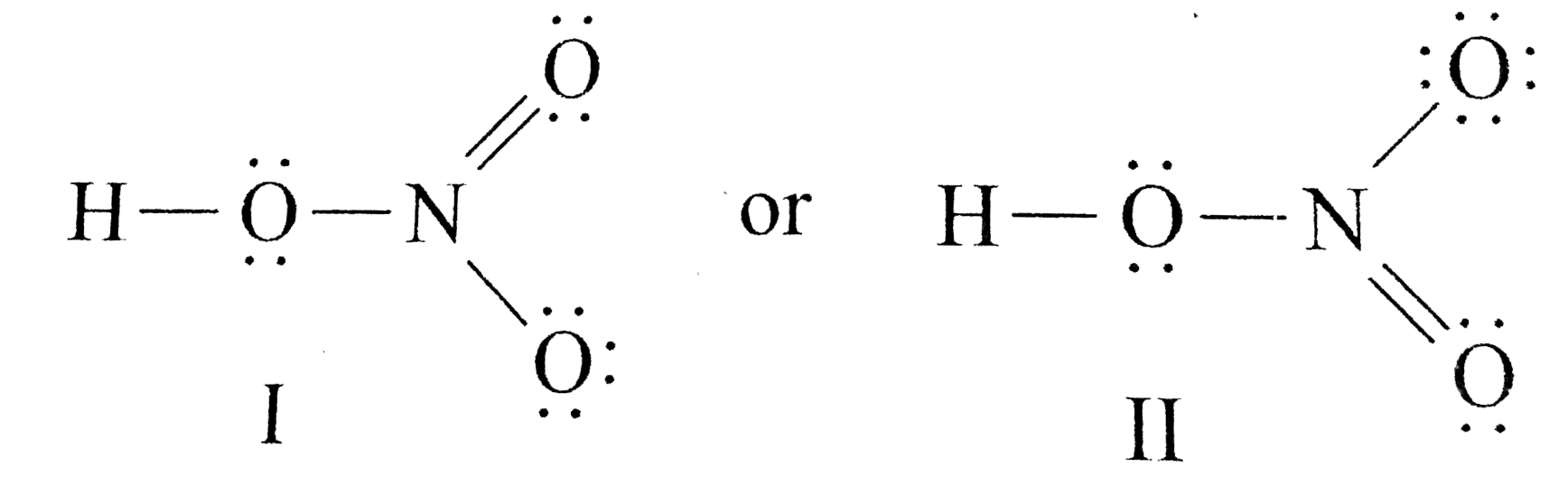
Draw The Lewis Structure Of Nitric Acid Hno 3
What Are The Lewis Structures For Hno3 Quora
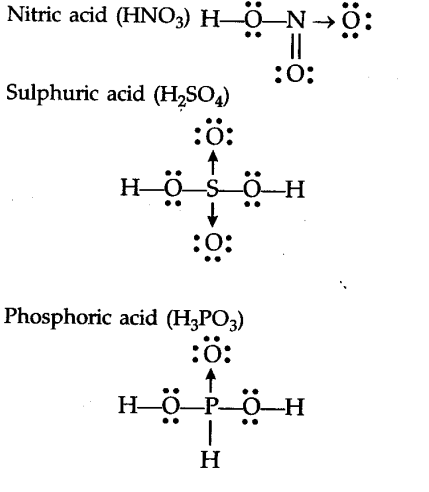
Draw The Lewis Dot Diagram Of Nitric Acid Sulphuric Acid And Phosphoric Acid Cbse Class 11 Chemistry Learn Cbse Forum
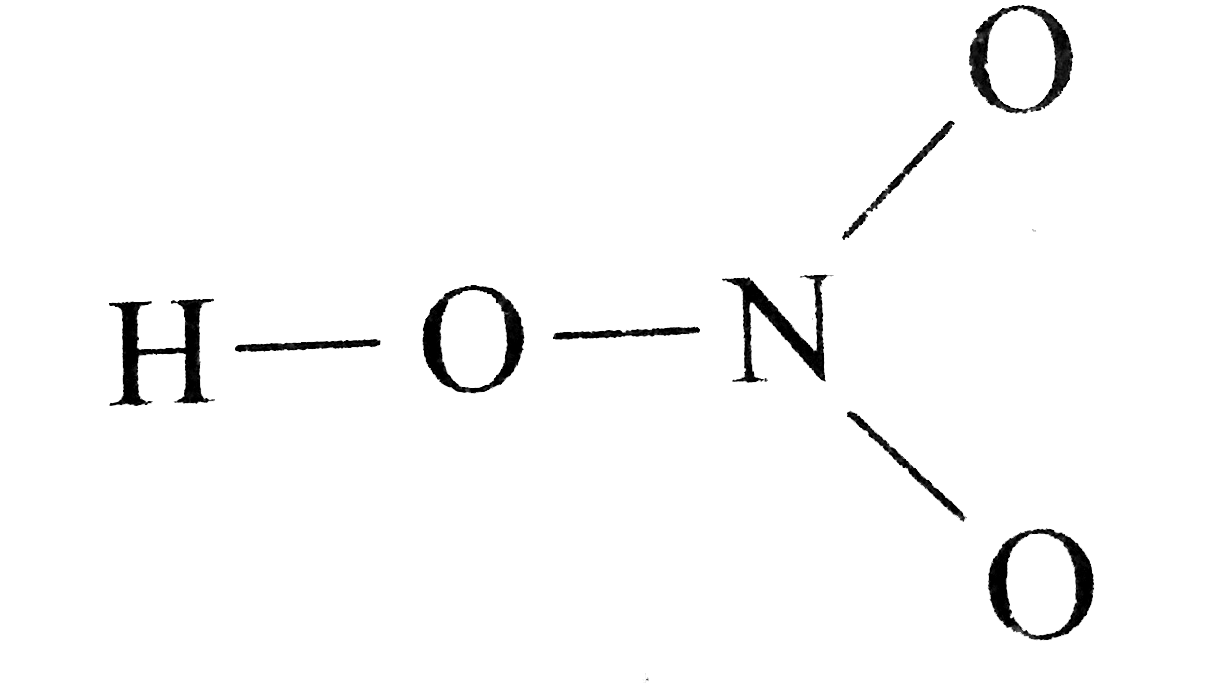
Draw The Lewis Structure Of Nitric Acid Hno 3

What Are The Lewis Structures For Hno3 Quora

Write Lewis Structure Of The Following Compounds And Show Formal C

Hno3 Lewis Structure Nitric Acid Youtube

