Lewis Structure For C2h2o2
Glyoxal has been identified as a disinfection byproduct in drinking water treated with chlorine dioxide and ozone 34. Structure properties spectra suppliers and links for.

Type Of Reaction For C2h2 O2 Co2 H2o Youtube
This is the C2H2Cl2 Lewis structure.
Lewis structure for c2h2o2. The liquid is yellow and the vapor is green. Carbon has 4 valence electrons two Carbons. Ap chem consider the molecules PF3 and PF5.
By signing up youll get. Bis the PF3 molecule polar or is it nonpolar. Glyoxal is a photochemical degradation product of aromatic and olefinic hydrocarbons 1.
Glyoxal is an organic compound with the chemical formula OCHCHO. Lewis structures of acetaldehydeethylene oxide and vinyl alcohol. Find the number of nonbonding lone pairs e-.
The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2. In COH 2 Lewis structure Carbon is less electron electronegative than Oxygen and goes in the center of the Lewis structure note that Hydrogen atoms always go on the outside. Find valence e- in all atoms.
Draw a Lewis structure for ketene C2H2O which has a carbon-carbon double bond. In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. Note the two H--- are bonded to the Carbon is a double bond and.
LEWIS FORMULAS STRUCTURAL ISOMERISM AND RESONANCE STRUCTURES CHARACTERISTICS OF LEWIS FORMULAS. Show all unshared electron pairs. Thus the electron-dot structure for acetonitrile is.
Draw the lewis structure for. It is a crystalline solid white at low temperatures and yellow near the melting point 15 C. Lewis structures also known as electron dot structures are named after Gilbert N.
You can draw a Lewis dot structure for any covalent molecule or coordination. See the Big List of Lewis Structures. Find octet e- for each atom and add them together.
Lewis structure of carbon dioxide. This set index page lists chemical structure articles associated with the same molecular formula. List of chemical structure articles associated with the same molecular formula.
In the Lewis structure for COH 2 there are a total of 12 valence. Additional examples of Lewis. None of the atoms bears a formal charge and all atoms have octets except for hydrogen atoms which have duets.
SiO3 -2 CNO- TeO4 -2 F2PPCl2 thanks. Lewis formulas are structures that show the connectivity or bonding sequence of the atoms indicating single double or triple bondsThey should also show any formal charges and unshared electrons that might be present in the molecule. It is the smallest dialdehyde a compound with two aldehyde groups.
Glyoxal is an organic compound with the chemical formula OCHCHO. Hydrogen has 1 valence electron but we have two Hydrogens. Explain C on the basis of bonding principles predict whether each of the following compounds exists.
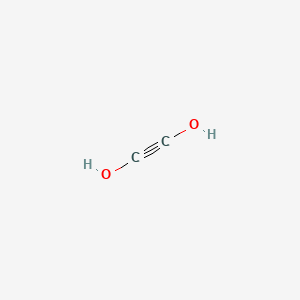
The molecular formula C2H2O2 may refer to. Drawing the Lewis Structure for COH 2. Chapter 1 Problem 26E is solved.
The liquid is yellow and the vapor is green. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure Ethyne or AcetyleneFor the C2H2 structure use the periodic table to find the total. This figure explains the bonding in a CO 2 molecule.
The reaction of cyclopentene with ozone in the atmosphere leads to the formation of glyoxal 2. Carbons the least electronegative and well put that in the center and we know Hydrogens always go on the outside. A step-by-step explanation of how to draw the C2H2Br2 Lewis Dot Structure 12-DibromoethyleneFor the C2H2Br2 structure use the periodic table to find the.
It is the smallest dialdehyde a compound with two aldehyde groups. Draw the most stable Lewis structure for C2H2O2. Step-by-step solution100 18 ratingsfor this solution.
Hydrogen has one valence electron carbon has four valence electrons and nitrogen has five valence electrons. What is the Lewis structure of c2h2o. Subtract step 1 total from step 2.
Lewis who described them in a 1916 article titled The Atom and the Molecule Lewis structures depict the bonds between atoms of a molecule as well as any unbonded electron pairs. Gives you bonding e-. Subtract step 3 number from step 1.
Plus Chlorine which is 7 and we have two Chlorines for a total of 24 valence electrons. Chemistry questions and answers. It is a crystalline solid white at low temperatures and yellow near the melting point 15 C.
For the COH 2 Lewis structure Hydrogen only needs two valence electrons for a full outer shell. Each O atom starts out with six red electrons and C with four black electrons and each bond behind an O atom and the C atom consists of two electrons from the O and two of the four electrons from the C. Describe the molecular geometry of each of the central atoms.
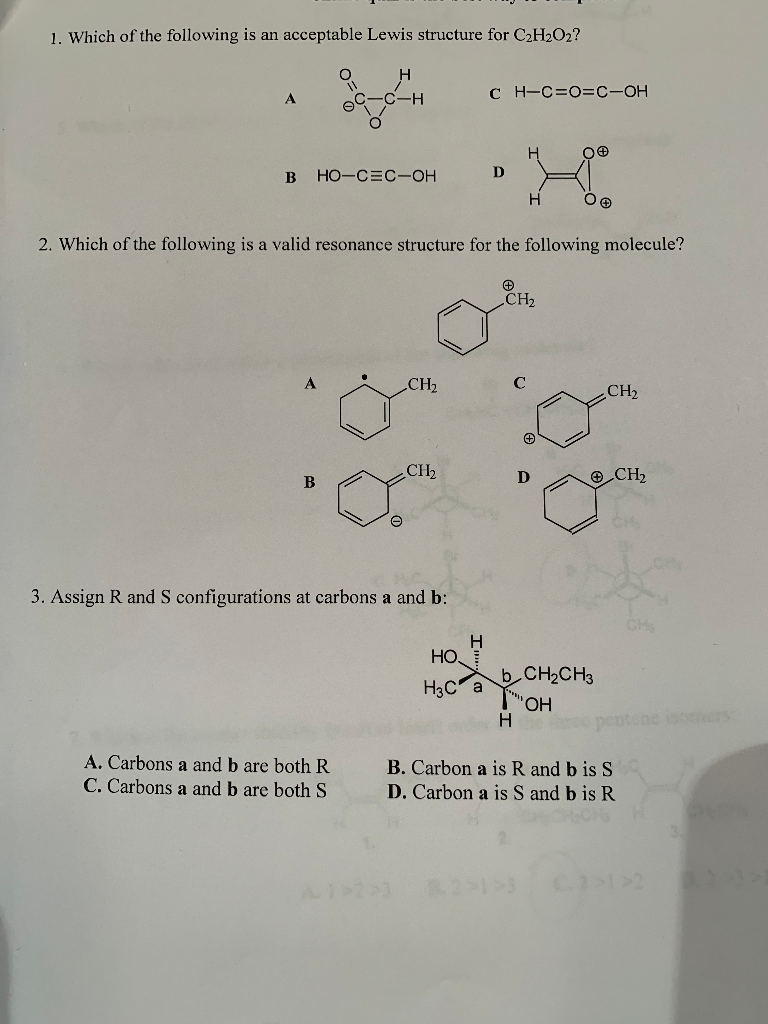
1 Which Of The Following Is An Acceptable Lewis Chegg Com
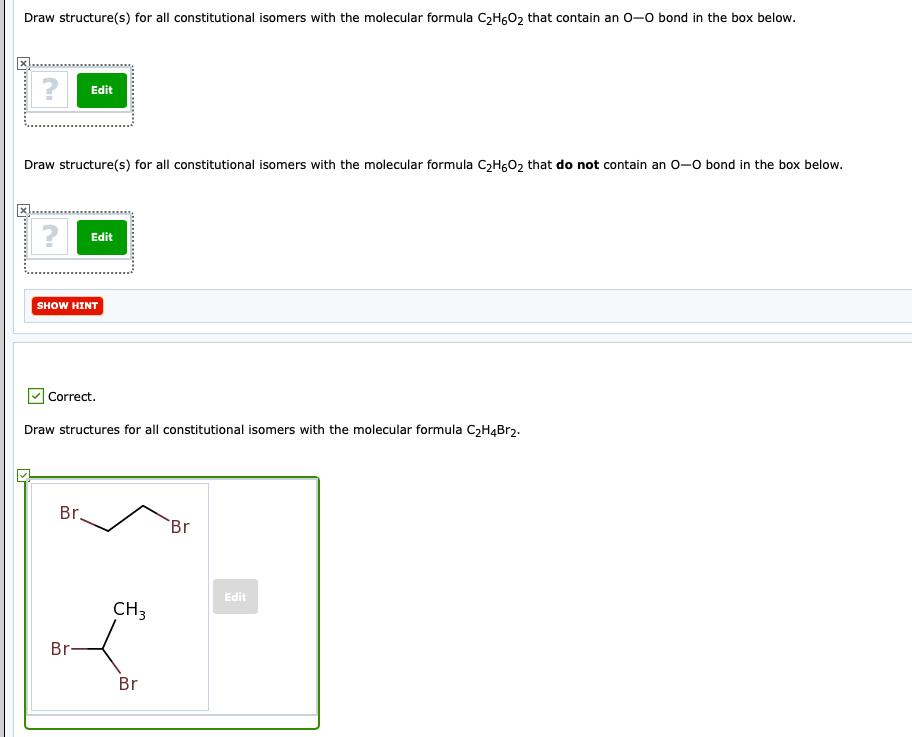
Draw Structure S For All Constitutional Isomers With Chegg Com
Https Kumarsir34 Files Wordpress Com 2016 11 Chemistry Class X For Sa Ii 2016 17 Pdf
Acetolactone C2h2o2 Chemspider
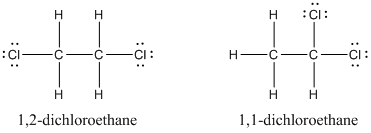
Solved Draw Lewis Structures For Each Molecular Formula A C2 H4 Chegg Com
Acetylenediol C2h2o2 Chemspider

What Do You Call The Number Of Atoms In A Molecule Chemistry Stack Exchange

Type Of Reaction For C2h2 O2 Co2 H2o Youtube
Https Repository Kaust Edu Sa Bitstream 10754 627380 1 Viewpageproof Cnf 9942 Pdf
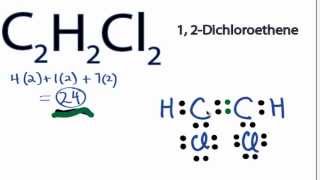
C2h2cl2 Lewis Structure How To Draw The Electron Dot Structure For C2h2cl2
Https Www Topperlearning Com Answer Draw Two Possible Structures Of The Compound With The Molecular Formula And Write Their Names Also Draw The Electron Dot Structures Of The Above Two C Zmj8oi22

Oneclass Draw Lewis Structure And Label If Resonance And Dipole If Polar Mf2cl2 1 C2h2o2 No3 1
Solved What Is Two Isomers For C2h2o2 And The Lewis Dot Structure Course Hero