Pf3 Lewis Structure
Solution for Formula Lewis structure Molecule or Electron group Molecular Bond Polarity Ion Type geometry geometry angle PF3 H2O PF3. Click to see full answer.

Predict Molecular Geometry Of Pf3 Phosphorus Trifluoride Youtube
Determine the number of bonding groups and the number of lone pairs around the central atom.

Pf3 lewis structure. Expert solutions for 41 The Lewis structure of PF3 shows that the central1192170. If we talk about the chemical composition of PF3 the molecule consists of one phosphorus atom and three fluorine atoms. Here in this post we described.
What is the electron domain geometry and molecular geometry of ph3. In the Lewis structure for PF 3 there are a total of 26 valence electrons. Each F-P bond contributes to the net dipole moment as a vector from the P to the F.
The molecular geometry unlike the electron geometry changes upon the introduction of lone pairs to the structure of a compound. Three pairs will be used in the chemical bonds between the P and F. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero.
In general the closer the formal charges are to zero the more stable the structure. Thus the first Lewis structure is predicted to be more stable and it is in fact the structure observed experimentally. To draw the Lewis structure of PF3 P F 3 we first count for the valence electrons of the compound.
The phosphorus trifluoride chemical formula is PF3. There are four electron groups on P. Mol mass of PF3 1 30 mol mass of P 3 189 mol mass of F 8796 gmol.
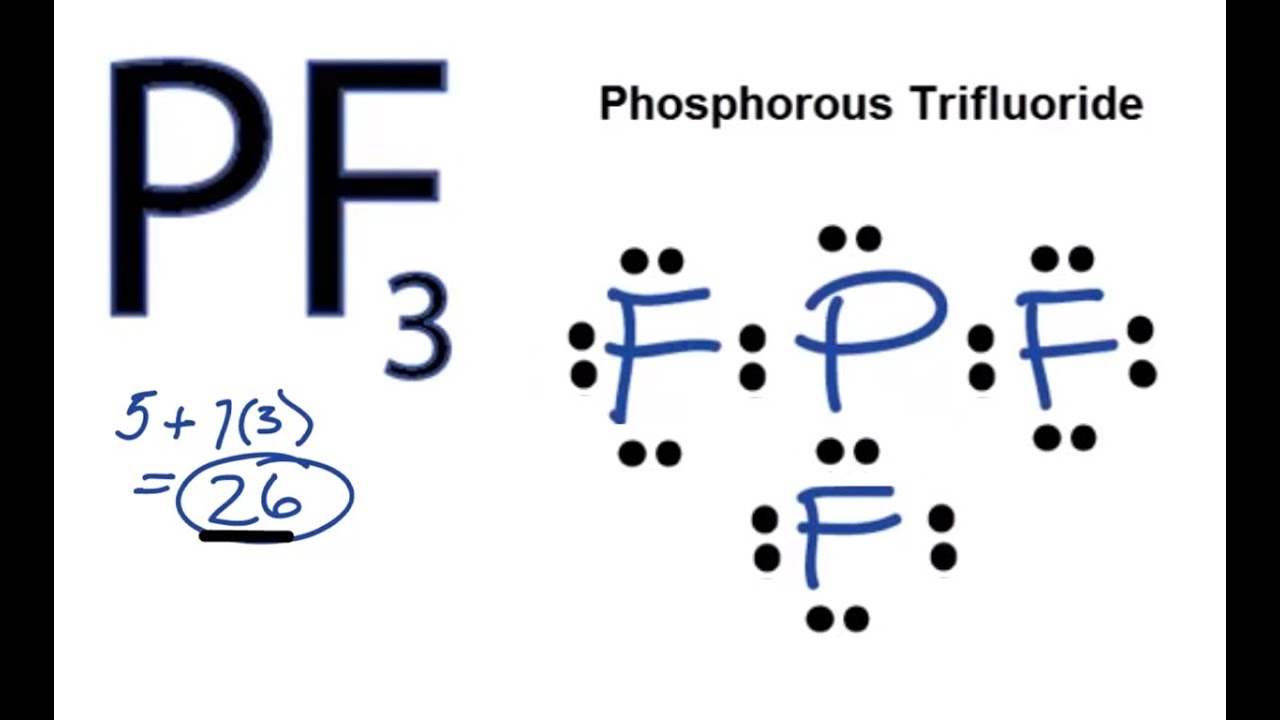
Phosphorus has 5 valence electrons while the 3 fluorine atoms have 21 valence electrons 7 for. Correspondingly what Vsepr shape is pf3. PF3 has 26 valence electrons.
Draw a Lewis structure for the molecule. The Lewis structure of PF3 shows that the central phosphorus atom has _____ nonbonding and _____ bonding electron pairs. The net dipole moment is the vector sum of the vectors along the three P-F bonds.
This problem has been solved. This E-mail is already registered as a Premium Member with us. We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule PF_3 because of its AX_3E status.
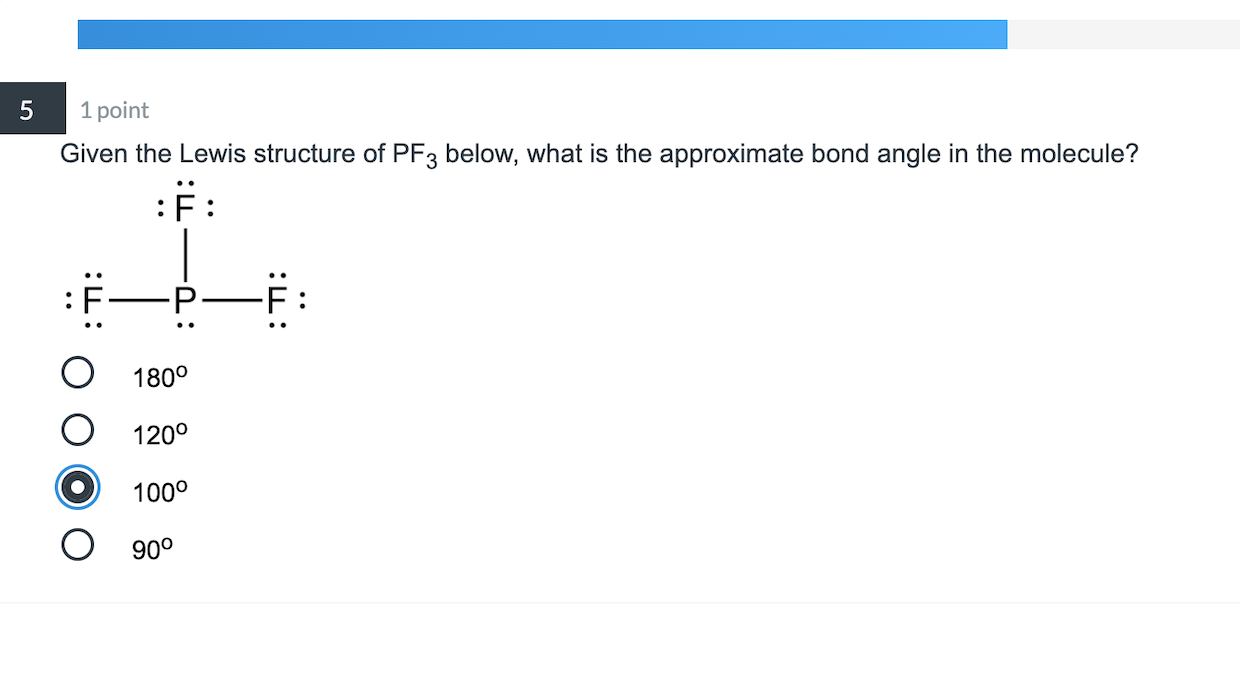
Three single covalent bonds are formed between the phosphorus and fluorine atoms which contributes to the presence of three strong sigma bonds and no pi bonds. In the PF 3 Lewis structure Phosphorus P is the least electronegative so it goes in the center. The F-P-F angles are 96 degrees.
Kindly login to access the content at no cost. The phosphorus is at the apex of a pyramid the base of the pyramid being an equilateral triangle with a fluorine atom at each vertex. This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will repel push away from each other in three dimensional space and this gives the molecules their shape.
Bond angle 1095 Because of the lone pair the bond angle will be less than 1095. Draw a Lewis structure for the molecule. Second place the valence electron on the iodine and hydrogen atoms.
PF3 has 26 valence electrons. The Lewis structure of the tetra-atomic phosphorus trifluoride PF3 molecule shows three fluorine atoms bonded to a single phosphorus central atom. C r -p -F Determine the total number of electron groups around the central atom.
2 2 1 3 1 2 3 3 3 1. First the valence electrons are placed around the carbon atom. There are three bonding groups and one lone pair.
Both Lewis structures have a net formal charge of zero but note that the formal charges on the first structure are all zero. Both of those are. The geometry of PH3 is pyramidal.
Drawing PF3 Lewis Structure is very easy to by using the following method. The valence electrons of Phosphorus are 5 and fluorine has 7 valence electrons in its outermost shell. See the answer See the answer See the answer done loading.
Phosphorus trifluoride F3P CID 62665 - structure chemical names physical and chemical properties classification patents literature biological activities. In this post we discussed the method to construct the CH3I Lewis structure. We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule P F 3 because of its AX3E status.
Lewis Structure Electron Groups. The molecular mass of PF3 is calculated as. VESPR stands for valence shell electron pair repulsion.

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube
Chemistry Learning Made Easy Pf3 Lewis Structure And Molecular Geometry Youtube
Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Answer The Molecular Geometry Of Pf3 Is B Clutch Prep

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

What Is The Molecular Geometry Of Pf3 Study Com
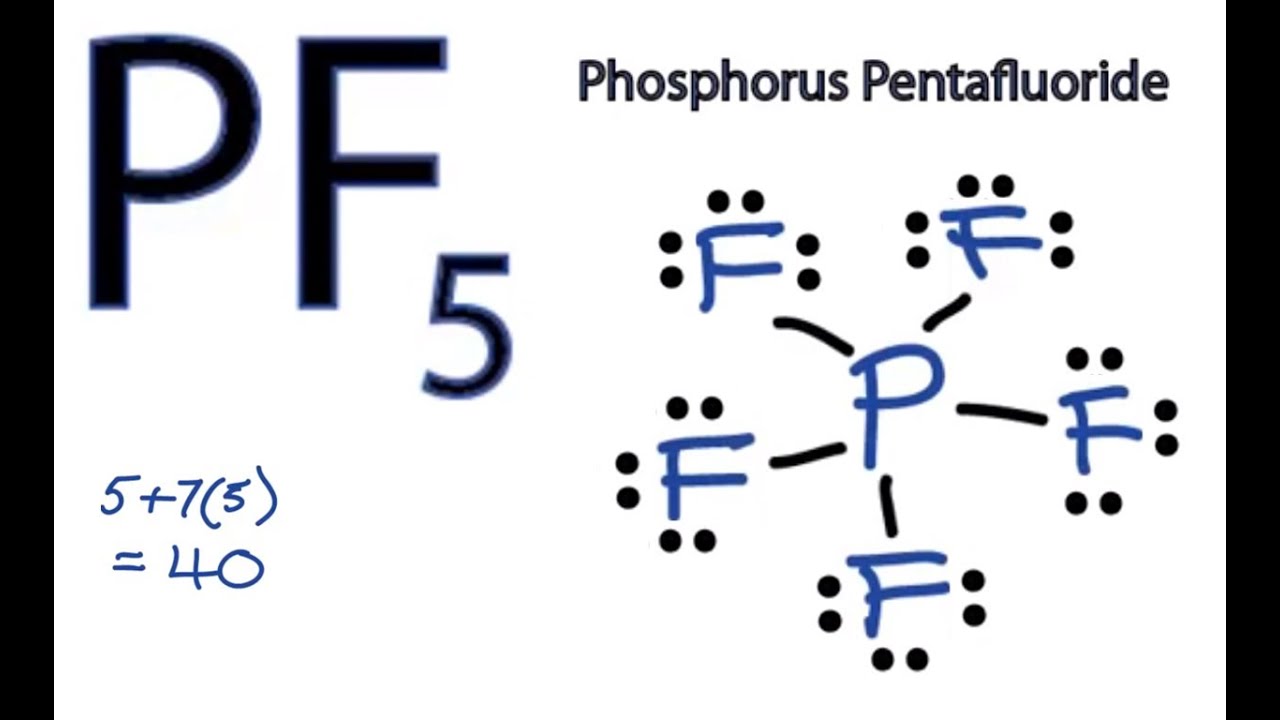
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Pf3 Molecular Geometry Shape And Bond Angles Youtube

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
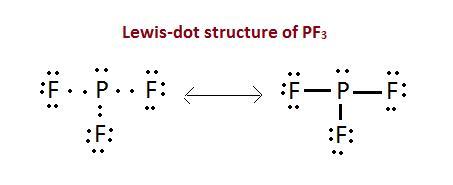
The Lewis Structure Of Pf3 Shows That The Central Phosphorus Atom Has Nonbonding And Bonding Electron Pair S
Lewis Dot Structure And Vsepr Model Sophat

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Lewis Structure For Pf3 Learn Lif Co Id
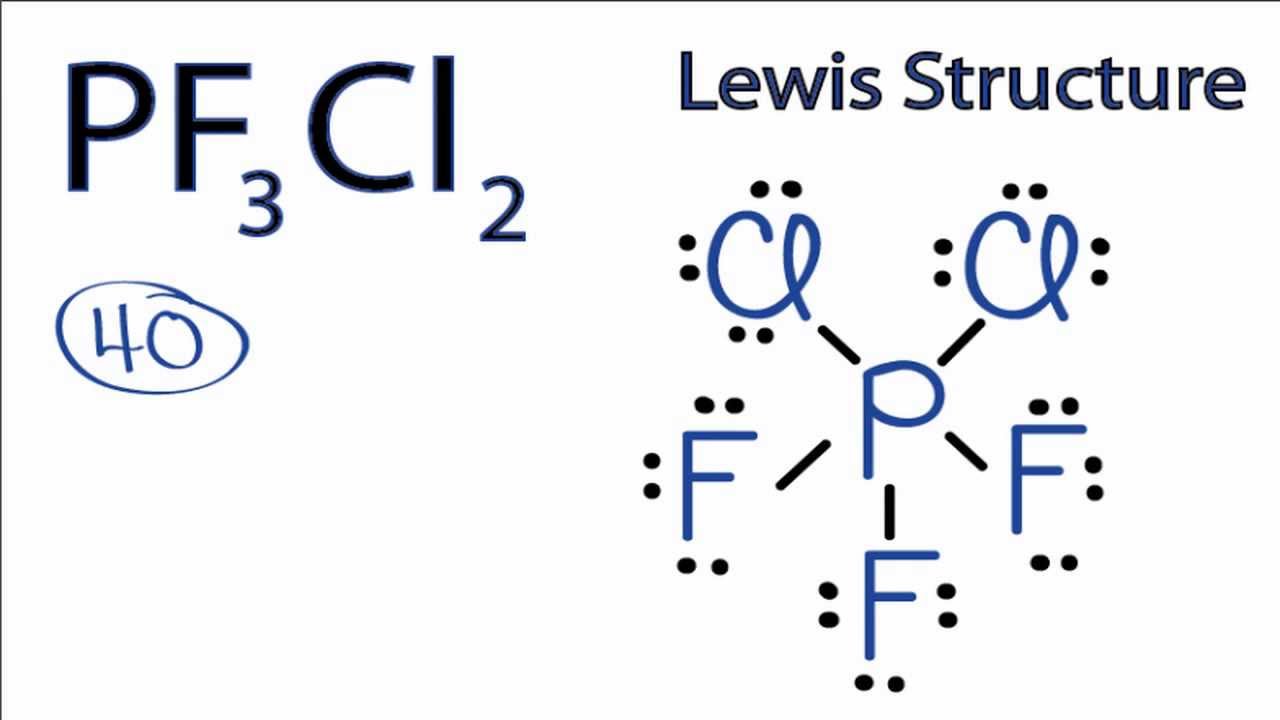
Pf3cl2 Lewis Structure How To Draw The Lewis Structure For Pf3cl2 Youtube

A What Is The Molecular Geometry Of Pf3 Clutch Prep
5 1 Point Given The Lewis Structure Of Pf3 Below Chegg Com
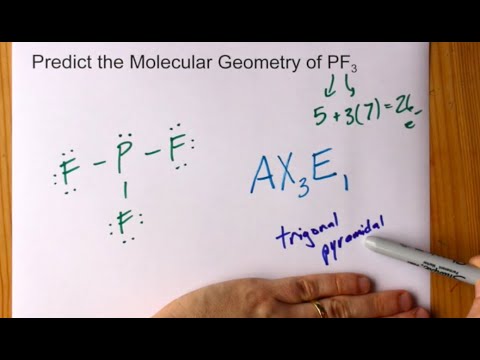
Predict Molecular Geometry Of Pf3 Phosphorus Trifluoride Youtube

Pf3 Molecular Geometry Shape And Bond Angles Youtube