So3 Lewis Structure Polar Or Nonpolar
SO3 Lewis Structure Sulfur Trioxide Welcome back to our channel and in todays video we are going to help you find out the Lewis Structure of SO3. Due to difference of electronegetivity between S and OSO3 should be polar.

Hybridization Of Of2 Oxygen Difluoride In 2021 Molecules Oxygen Things To Come
- lewis structure drawing - bonding electrons - nonbonding electrons - hybridization - AXE notation - molecular geometry - polar or nonpolar - resonance - isomers - wedge and dash drawing.

So3 lewis structure polar or nonpolar. For better understanding also check out an article written on SO3 Lewis Structure and its Molecular Geometry. The ammonia NH3 molecule has an sp3 hybridization but not tetrahedral geometry. - SF3Cl3 shows sp3d2 hybridization.
They all are having the same number of bonds and lone pair electrons. Are SO3XeF2IF2- polar or nonpolar. Hi guys welcome back to our channel and in todays video we are going to help you determine if BCL three is a polar or nonpolar molecule.
Chemistry questions and answers. Therefore the total number of bonds becomes three forming a bond angle of 120 degrees that cancel the polarity. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search.
First the valence electrons are placed around the carbon atom. According to Vallence bond theory what is the hybridization of all atoms in SO 3. The central atom in the trigonal planar molecule bonded to the three atoms and has no electron pairs.
Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. In this formula all atoms of oxygen are symmetrical. This results in a folded bent structure so the folded bent structure distributes the charge unevenly across the molecule including a.
- It contains Xe with 1 e- pair O with 2 e- pairs F with 3 e- pairs. How it is polar or nonpolar. If you look at the Lewis structure for SO3 it appears to be a symmetrical molecule.
Draw the Lewis structure for SO3. But in SO3 the valance electrons are equally sharedHence it is non polar. Complete the following for BrF3 SF4 IF4 SO3-2 XeF2 and SF2.
So to determine is polarity we first need to look at its lowest structure and then ah check the differences of electro negativities of the atoms in all in the structure and lastly check if there is a net diaper movement in this molecule. The sulfur trioxide SO3 is a nonpolar molecule because the shape of the SO3 is trigonal planar. Polarity comes from due to difference of electronegetivity and unequal sharing of valance electrons in a compound.
The Phosphorous trichloride PCL3 is a polar molecule due to a lone pair of electrons at the top of the molecule which leads to the electron-electron repulsion. Second place the valence electron on the iodine and hydrogen atoms. However to determine if SO3 is polar we need to look at the molecular.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Therefore SO3 is a nonpolar molecule. So3 2- sulfite ion is Polar Ill tell you the polar or nonpolar list below.
You can go through lewis structure of H2O molecule and SF4 polar or nonpolar for more details on the Octet rule. Some of the professionals also have the same confusion. Determine the number of electron groups the electron geometry and the molecular shape.
Learn to determine if SO42- is polar or nonpolar based on the polarity between bonds and the molecular geometry shapeIons like SO42- sulfate are someti. Answer SOF4 Thionyl tetrafluoride is Polar What is polar and non-polar. In this post we discussed the method to construct the CH3I Lewis structure.
Below is the lewis structure of the Ammonia molecule for better understanding for students. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. Write Lewis dot structures for covalent molecules Molecule Number of valence electrons Lewis dot structure Geometry Polar or Non-polar SO3.
- XeO2F2 shows sp3d hybridization with seesaw structure. Here is the answer in the simplest explanation SO 3 is NON-POLAR. - SF3Cl3 is polar molecule with S pulling electron clouds towards it.
SF3Cl3 is a polar molecule. The bond formation of Sulfur trioxide depicts the symmetry of it which makes it nonpolar. In the case of Sulfur trioxide SO3 the trigonal planar shape having bonds at 120 degrees with each other that cancels out the polarity of each bond.
Lewis structure of XeOF2 -. Lewis structure of NH3 molecule follows Octet rule. Add formal charges and bond dipoles to all lewis dot structures.
Many students have the question Is SO 3 polar or nonpolar. Question Is SOF4 polar or nonpolar. Why SO3 is nonpolar.
List molecules polar and non polar. Describe the orbitals that overlap to form all bonds in SO3.

Ch3cl Lewis Structure Chloromethane In 2021 Lewis Molecules Methylation

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules

Is So3 Polar Or Nonpolar Youtube

Chemistry Intermolecular Forces Polar Bonds And Polarity Teaching Chemistry Chemistry Classroom Chemistry Education

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

So3 Molecular Geometry Lewis Structure And Polarity Explained

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

So3 Molecular Geometry Lewis Structure And Polarity Explained

So3 Molecular Geometry Lewis Structure And Polarity Explained

So3 Lewis Structure Sulfur Trioxide Youtube

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
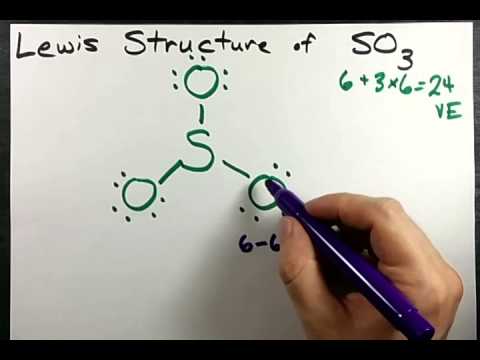
Lewis Structure Of So3 Sulfur Trioxide Youtube

What Is The Difference Between The Lewis Structure Of So3 Vs So3 2 Quora

Ccl4 Lewis Structure Carbon Tetachloride In 2021 Carbon Molecule Molecules Lewis
So3 Molecular Geometry Lewis Structure And Polarity Explained

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots
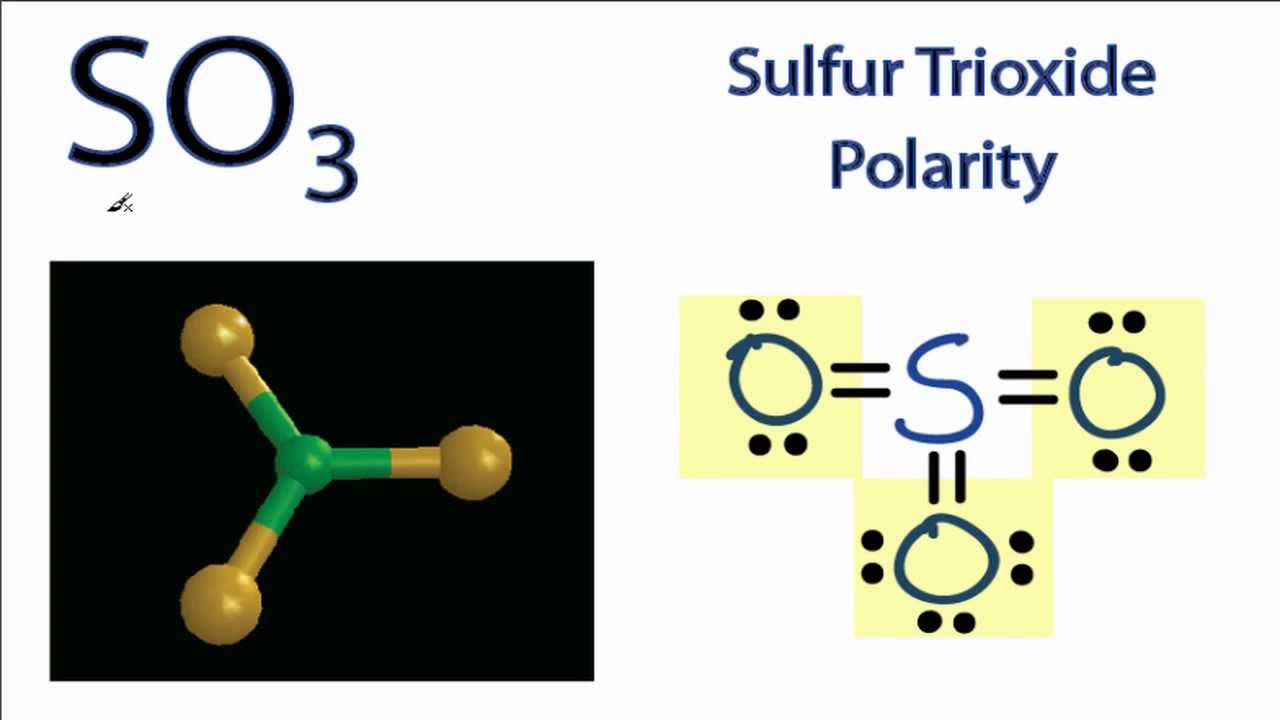
Is So3 Polar Or Nonpolar Youtube

