What Is The Lewis Structure Of Cocl2
Write these charges next to the atoms in the Lewis structure. COCl2 Lewis Structure Molecular Geometry Hybridization and Polarity COCl2 is a chemical compound known by the name phosgene.

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar
Cl 6 electrons from lone pairs plus 1 electron from a bond with C 7 electrons.
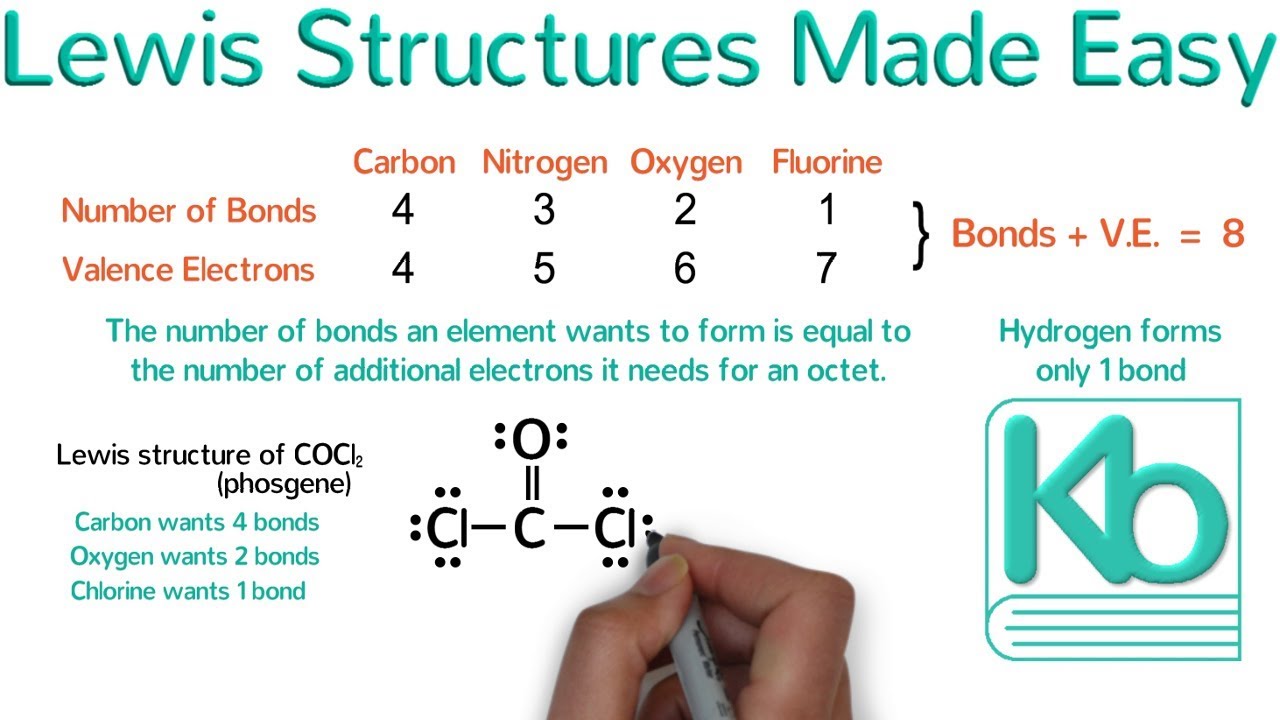
What is the lewis structure of cocl2. O 4 electrons from lone pairs plus 2 electrons from bonds 6 electrons. Its Lewis structure can be drawn 3 ways. Lewis dot structure of CO Cl 2.
Cl 6 electrons from lone pairs plus 1 electron from a bond with C 7 electrons. Write these charges next to the atoms in the Lewis structure. What is the Lewis structure for COCl2.
Alternatively a dot method can be used to draw the lewis structure of COCl 2. A double bond represents a slightly larger electron domain which disrupts the ideal 120 Cl-C-Cl bond angle making it smaller. The first with a double bond between carbon and oxygen the second with a double bond between carbon and one chlorine and the third with a double bond between carbon and the other chlorine.
In the Lewis structure for COCl 2 there are a total of 24 valence electrons. It is readily soluble in water alcohol and acetone. Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of 9892 grammol.
LIIV v Meo What is the relationship between the two given structures. The Lewis Dot Structure for COCl 2. What is the Lewis dot structure of COCl2.
The most stable structure for COCI2 is Structure A. C 0 lone pairs plus 4 electrons from bonds 4 electrons. CoCl2 is a crystalline solid that is sky-blue in color.
The Lewis dot structure for any molecule can be found by following a general set of rules consisting of 5 or sometimes 6. The Lewis structure of the CoCl2 molecule shows that there is a double bond between the O and the central C atom. A step-by-step explanation of how to draw the HOCl Lewis Structure Hypochlorous AcidBecause HOCl is an acid well put the Hydrogen atom on the outside of.
It is non-flammable in nature and bears a suffocating odor. O 4 electrons from lone pairs plus 2 electrons from bonds 6 electrons. What is the correct Lewis structure for CoCl2.
Youll need to form a double bond between the Carbon and Oxygen to complete the octet on the Carbon atom. Calculate the total valence electrons in COCl 2 molecule. Both CCl bonds are polar due to the difference in electronegativity of C and Cl.
What are the configurations E or 2 of the indicated double bonds. In the COCl 2 Lewis structure Carbon is less electron electronegative than Oxygen and goes in the center of the Lewis structure note that Hydrogen atoms always go on the outside. CoCl2 with an LD50 of 11 mM was about five times more potent than NiCl2.
Bereken voor de HOCl Lewis-structuur het totale aantal valentie-elektronen voor het HOCl-molecuul. Newer Post Older Post Home. The chemical formula CoCl2 represents Cobalt II Chloride.
C 0 lone pairs plus 4 electrons from bonds 4 electrons. Chemical Bonding Lewis structure Lewis symbol. CH I C 0.
CoCl2 Lewis Structure Molecular Structure Hybridization Bond Angle and Shape. C4O6Cl2x714 Total24 Put carbon in the center. The Lewis structure of the COCl2 molecule shows that there is a double bond between the O and the central C atom.
It is an inorganic compound that comprises Cobalt and Chlorine atoms. Since the double bond can be placed in more than one place without rearranging the atoms COCl2 exhibits resonance. Which is the most stable Lewis structure for COCl2.
What is the Lewis structure of CoCl2. Immortalized alveolar epithelial type II cells were incubated for 4 hr with various concentrations of either NiCl2 CoCl2 or NiCl2 and CoCl2 together and cell viability assessed 24 hr later. Nadat je hebt bepaald hoeveel valentie-elektronen er in HOCl zijn plaats je ze rond het centrale atoom om de octetten te voltooien.
Lewis dot structure 2 is the more probable most stable since there is no charge separation. Er zijn in totaal 14 valentie-elektronen in de Lewis-structuur voor HOCl. A double bond represents a slightly larger electron domain which disrupts the ideal 120 CI-C-Cl bond angle making it smaller.
The LD50 for NiCl2 was 57 mM. Therefore the dot resonance structures of COCl 2 Lewis structures of COCl2 are as follows. Expert Answer 97 111 ratings The COCl2 molecule has 3 areas of electron repulsion around the central C atom so the shape is trigonal planar.

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Math Equations Lewis Molecules

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo High School Chemistry Teaching Science Organic Chemistry Study

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo High School Chemistry Teaching Science Organic Chemistry Study

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar