Why Is Xef2 Nonpolar
As there are fluorine molecules on both the side of the central atom there is no dipole moment and hence there is no polarity. Since they are the same atoms they have the same electronegativity electrons pulling power.

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules
In the XeF2 compound the electronegativity difference between fluorine 398 and Xe 26 is 398-26 138 which shows the Xe-F bond is polar according to the Pauli scale.
Why is xef2 nonpolar. Why is CHCl3 a polar molecule. This is because XeF4 has an octahedral symmetric geometry. Why is XeF2 a nonpolar molecule.
Otherwise it is polar. SCN- ion consists of 1 sulfur 1 carbon and 1 nitrogen atom. Having one on the left and another on the right makes the molecule symmetric thus cancelling the dipole moment making XeF2 non-polar.
In XeF2 molecule two fluorine atoms are arranged symmetrically on the outside with the central atom Xenon in the middle. XeF2 is nonpolar due to the symmetric arrangement of the bonded pairs of electrons. This explains why XeF2 has a linear molecular shape.
The polarity induced on four Xe-F bonds are cancel each other and the. In CHCl3 the molecular shape is tetrahedral meaning that the H and the three Cl atoms will occupy the vertices of a triangular based pyramid around the central C atom. CCl4 is an example of a nonpolar molecule.
XeF2 is nonpolar due to the symmetric arrangement of. There is no net dipole moment in the compound due to the arrangement of the valence electrons in symmetry. So the shape of XeF2 ion is linear.
The compound XeF2. Hope that helps. Having one on the left and another on the right makes the molecule symmetric thus cancelling the dipole moment making XeF2 non-polar.
Detailed Explanation As we have already known there are certain conditions for a molecule to be polar or nonpolar in the above definitions of the polar molecule and nonpolar molecule. Why is XeF2 nonpolar. According to the VSEPR theory The molecular geometry of the molecule is linear.
Nonpolar molecules are considered as pure covalent bond because it forms by equal sharing of electrons between the atoms in a compound and this is what covalent bond is known for. Is CCl4 polar or nonpolar. According to the VSEPR theory The molecular geometry of the molecule is linear.
Theoretically XeF2 may be called to have trigonal bipyridal structure. What kind of bond is XeF4. When other atoms substitute for.
XeF4 is a nonpolar molecule despite four individual Xe-F bonds are polar. One more case for the nonpolar molecule if the molecules have symmetrical structure so that the induced partial charges on the individual atoms nullify each other and the net dipole moment becomes zero. If the net dipole moment is zero it is non-polar.
Hence Xenon Difluoride is nonpolar as there is no polarity observed in the molecule. However Xe-F bond is polar because the electronegativity of Xe and F is different but the polarity of both Xe-F bonds gets canceled by each other resulting in a nonpolar XeF2 molecule. XeF2 is nonpolar due to the symmetric arrangement of.
XeF2 is nonpolar due to the symmetric arrangement of the bonded pairs of electrons. The molecule has a single bond between sulfur and carbon having negative charge on sulfur as it accepts one electron to complete its octet. As there are fluorine molecules on both the side of the central atom there is no dipole moment and hence there is no polarity.
Why is SCN Nonpolar in nature. As there are fluorine molecules on both the side of the central atom there is no dipole moment and hence there is no polarity. XeF2 is nonpolar in nature because of its linear-shaped geometry having fluorine atoms symmetrically on both sides of the xenon atom.
In XeF2 there are two fluorine atoms are bonded with a central xenon atom. It has a symectrical bonding so it can be considered nonpolar just on that yet it. The same conclusion can be derived from.
Xe has one F on the left and another on the right. The four bonds of carbon tetrachloride CCl4 are polar but the molecule isnonpolar because the bond polarity is canceled by the symmetric tetrahedral shape. So in Xe F2 Xe with sp3d hybridization has three lone pairs and two bond pairs.
The same things apply here which makes XeF2 a molecule. According to the VSEPR theory The molecular geometry of the molecule is linear. Read the full answer The molecule has octahedral electron geometry and square planar molecular geometry.
XeF2 is nonpolar due to the symmetric arrangement of the bonded pairs of electrons. Why is XeF2 nonpolar. The central atom of XeF2 Xe has a total of 10 electrons localized in the form of 4 as Xe-F bonding and 6 as non-bonding pairs.

Xef2 Structure All Knowledge About Xef2 Molecular Geometry Knowledge Vsepr Theory

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Xef2 Structure All Knowledge About Xef2 Molecular Geometry Knowledge Vsepr Theory

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Is Chf3 Polar Or Nonpolar Fluoroform In 2021 How To Find Out Molecules Hydrogen Atom

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

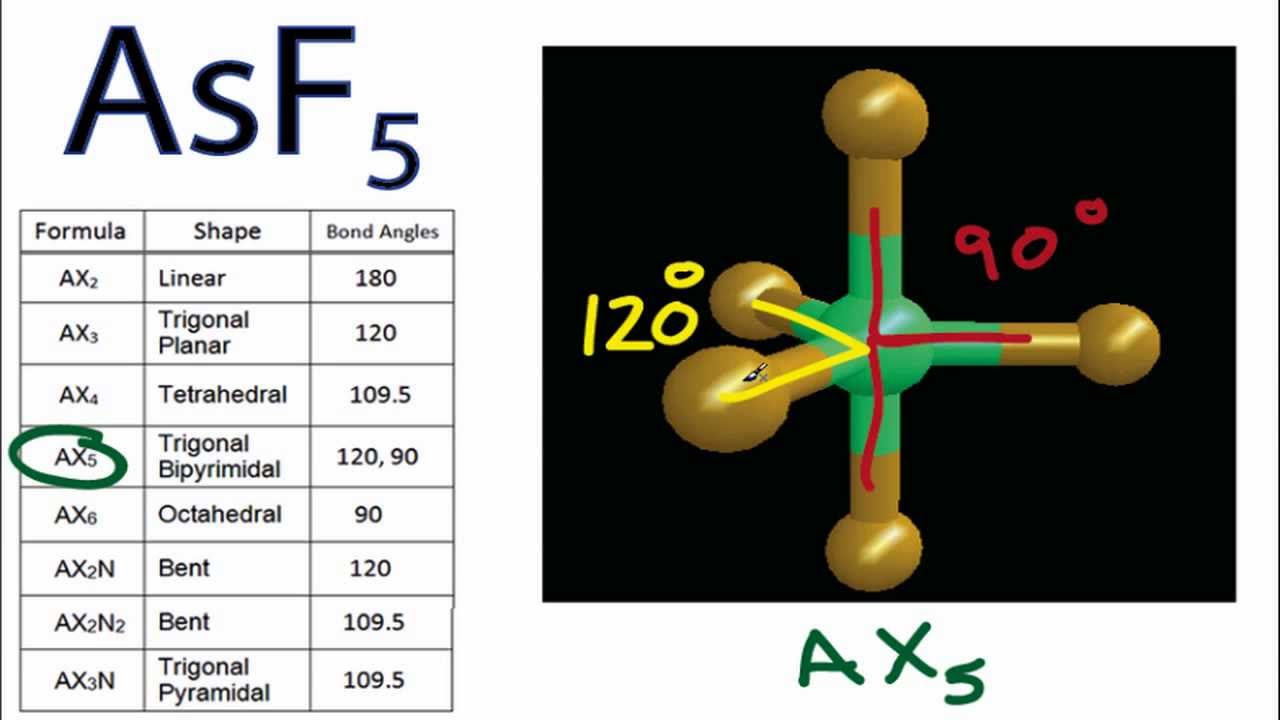
Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar

Pf5 Molecular Geometry Shape And Bond Angles Molecular Geometry Geometry Shape Molecular

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Is Cs2 Polar Or Nonpolar Carbon Disulfide In 2021 Math Equations Chemical Formula Molecules

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons

Is Pf5 Polar Or Non Polar Phosphorus Pentafluoride In 2021 Chemical Formula Molecules Phosphorus

Is Sf4 Polar Or Non Polar Sulfur Tetrafluoride In 2021 Math Equations Chemical Formula Molecules

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula

Xef2 Lewis Structure Xenon Difluoride In 2021 Lewis Molecules Math Equations

