Ammonium Hydroxide Lewis Structure
The aqueous solution is analyzed for cyanide ion by silver nitrate titrimetric method or an ion-selective electrode method and ammonia is measured by titration or electrode technique. Ammonium hydroxide chemical solution ammonia water fisher acros acs organics plus certified reagent bottle analysis identifiers glass fishersci.

Nh4oh Lewis Dot Structure Ammonium Hydroxide Youtube
This can be represented by the following equilibrium reaction.
Ammonium hydroxide lewis structure. 1-Butanaminium NNN-tributyl- hydroxide 11 tetran-butylammonium hydroxide. Is there a coordinate covalent bond as well in the structure. Ammonia is a colorless gas with a distinct odor.
NH3 H2O NH4 OH. Tetrabutylammonium hydroxide 40 solution in water. Jeremy8572 Sat 12082007 - 1434.
Can someone please help me. They follow the duet rule 2 electrons. Does the ammonium ion hydrogen bond with water.
Concentration of ammonia ranges up to approximately 30. Is there any way of representing a dativecoordinate covalent bond. Formal charge over ammonium ion is 1.
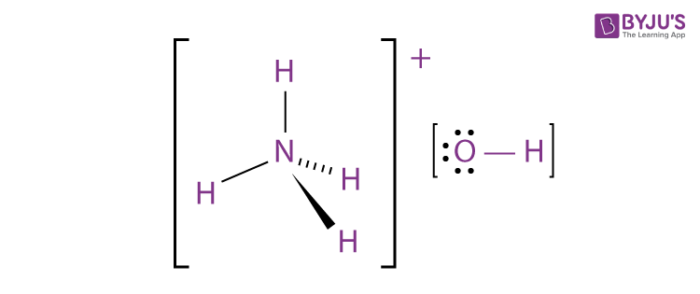
Write the Lewis structure for the ammonium ion NH 4. This can be expressed by the equation NH 3 H 2 0 NH 4 OH. The exception of course being the hydrogens.
Ammonia is a colorless gas with a chemical formula NH 3. SO2 Lewis structure sulfur dioxide electron dot structure is that type of diagram where we show the total 18 valence electrons of SO2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots. Now we have to determine the central atom in NH₃The central atom is that kind of atom that is single or that has lower electronegativityIn case of NH₃N is the central atom and H is the outer atomRemember that hydrogen is always the outer atom.
After counting the valence electrons we have a total of 9 5 from nitrogen 41 from each hydrogen 9. Average mass 35046 Da. The molecular mass of ammonium is 18039 gmol1.
Ammonia vapors which arise from the solution irritate the eyes. Ammonium hydroxide is a solution of ammonia in water. Uses of NH4 The compounds formed by the ammonium ions are widely used in the production of fertilizers.
When ammonia is dissolved in water the water molecules donate a proton to the NH 3 molecule. The ammonia molecule has a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory VSEPR theory with an experimentally determined bond angle of 1067. Household ammonia is NH 3 in water or ammonium hydroxide.
It consists of hydrogen and nitrogen. In this case because the ammonium ion is donating a proton it is. The ammonium compounds have great use in medicines and in textile industries.
Its acidity is 925 PKA. Ammonium cyanide may be analyzed by heating the salt and trapping the decomposed products. Hydrogen cyanide and ammonia in water at low temperatures.
70 More Lewis Dot Structures. NH3 8 valence electrons. It has a role as a food acidity regulator.
Monoisotopic mass 35037113 Da. Lewis Dot of Ammonia. Hydrogen atoms are always placed on the outside of the molecule so nitrogen should be the central atom.
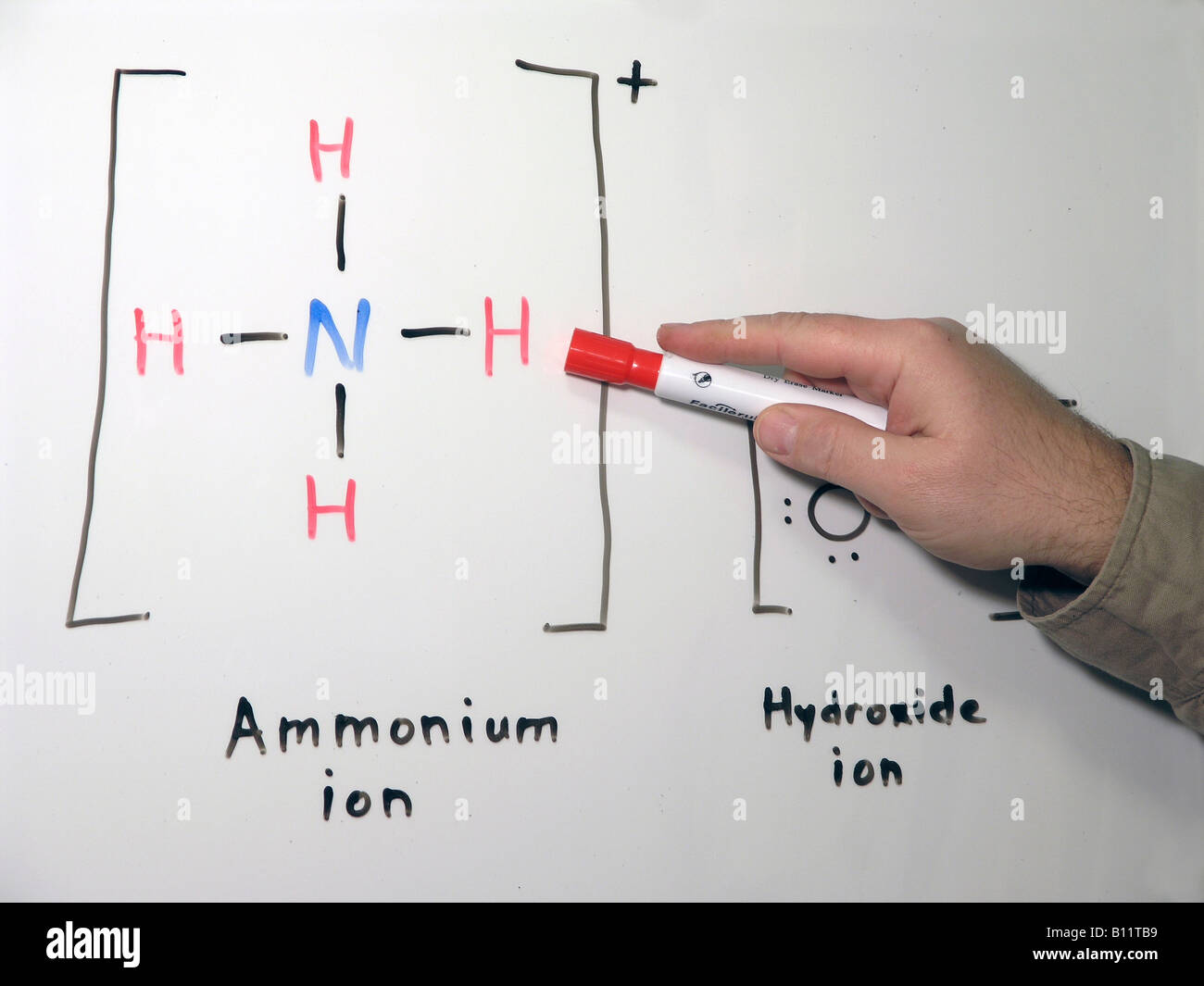
This inorganic compound has a pungent smell. The hydrogen on the ammonium ion NH4 can go back to the hydroxide ion OH- to form NH3 and H2O ammonia and water again. The molecular shape of this cation is tetrahedral.
Lewis structure of ammonium hydroxide lewis structure of ammonium hydroxide. Ammonia solution used as a cleaning solution is ammonia gas NH 3 which is dissolved in water. I need to come up with the lewis structure of ammonium hydroxide and no matter how hard I try I cant figure it out.
The central nitrogen atom has five outer electrons with an additional electron from each hydrogen atom. Lewis ammonium structure ion nh4 structures hydroxide oh shape atom chemical chloride dot covalent ions polyatomic bond charge electrons example. Lewis Structure of ammonia gas formula NH 3.
What would be the lewis structure for ammonium ion. Stability of Sulfides - backbonding. Lewis structure dot ammonium hydroxide nh4oh.
Molecular Formula H 5 NO. Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule. Ammonium hydroxide appears as a colorless aqueous solution.
You are familiar with the pungent odor of ammonia gas which is also used as a respiratory stimulant smelling salts. Ammonia and hydroxide ion - Lewis basicity. NH3 Lewis structure Setup Step-2.
In its concentrated form it is dangerous and caustic. In its aqueous form it is called ammonium hydroxide. This leads to the formation of an ammonium cation whose chemical formula is NH 4 and a hydroxide ion OH.

Nh4cl Lewis Dot Structure Ammonium Chloride Youtube

Nh4 Lewis Structure How To Draw The Dot Structure For Nh4 Ammonium Science Chemistry Molecular Geometry Chemistry

Nh4oh Lewis Dot Structure Ammonium Hydroxide Youtube
Ammonium Hydroxide Nh4oh Structure Properties Uses Of Ammonium Hydroxide

Nh4cl Lewis Dot Structure Ammonium Chloride Youtube

Electron Dot Structure For Nh4oh Tex Lt Marquee Gt Answer Asap Tex Brainly In

How To Draw Lewis Dot Structures For Ionic Compounds Bonds Youtube

How Is The Shape Of Ammonium Ion Determined Quora

Module 1 Electronic Structure And Covalent Bonding Lecture 1 Structure And Bonding I 1 2 3 4 5 6 7 1 4 Representation Of Structure Lewis Structures Represent The Valence Electrons Of An Atom As Dots By Using Lewis Structures One Can Recognize If Any
Ammonium Hydroxide H5no Chemspider

Video Lewis Dot Structure For Nh4

What Type Of Bond Does Nh4oh Have Quora
Ammonium Carbonate Nh4 2co3 Pubchem
Page 2 Ammonium Hydroxide High Resolution Stock Photography And Images Alamy

Lewis Structure Of Nh4oh Brainly In
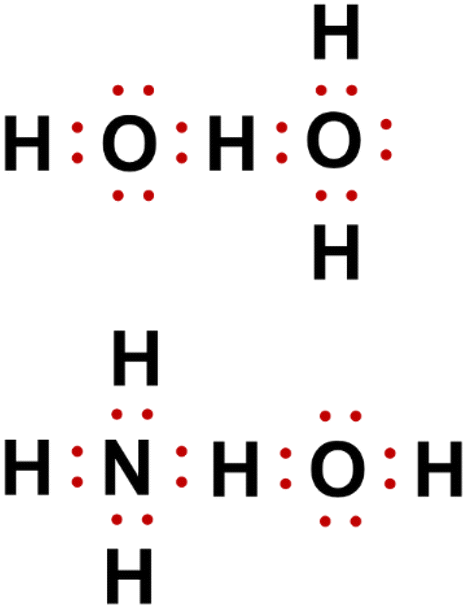
Figure 1 One Hundred Years After The Latimer And Rodebush Paper Hydrogen Bonding Remains An Elephant Springerlink

Oh Lewis Structure How To Draw The Lewis Dot Structure For The Hydroxide Ion Youtube