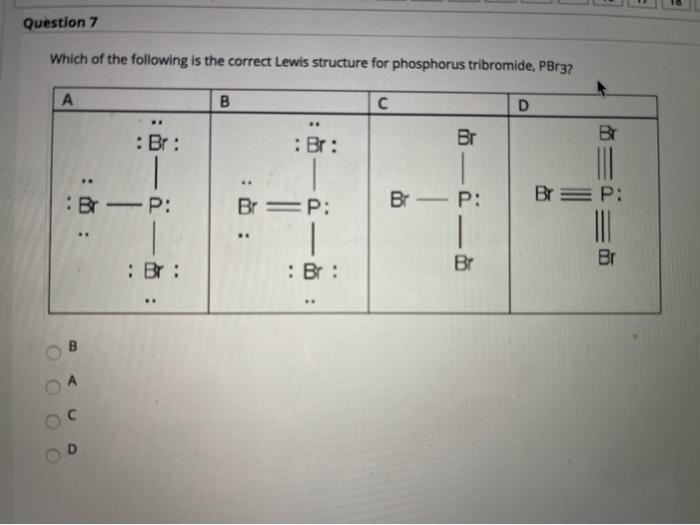
Pbr3 Lewis Structure Formal Charge
This is a placeholder for your sticky navigation bar. A step-by-step explanation of how to draw the PBr3 Lewis Dot Structure Phosphorus TribromideFor the PBr3 structure use the periodic table to find the tota.
Calculate The Formal Charge Of Each Of The Molecules Chegg Com
This is how we calculate the formal charge.

Pbr3 lewis structure formal charge. Draw the Lewis structures of each molecule. There has been one report of PBr3 effectiveness on real-scale flames indicating an effectiveness two orders of. Determine lewis structure pbr3 lone pairs electrons phosphorus ato.
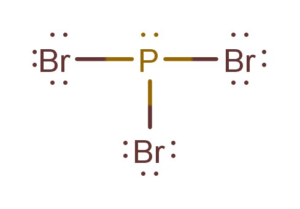
In c the nitrogen atom has a formal charge of 2. Include formal charges where appropriate. PBr3 Lewis StructureLewis Structure of PBr3 Phosphorus TribromideDraw Lewis Structure for PBr3Phosphorus Tribromide Lewis Structure.
Here in this post we. Next lets do the Boron. Structure b is preferred because the negative charge is on the more electronegative atom N and it has lower formal charges on each atom as compared to structure c.
A PBr3 b SiF4 c BF4 Question. Six over 2 equals3-3 0. Yes P has a formal charge of 1 however remember u can drag one of the lone pairs of the Br down to fill in the octet giving the Br the 1 charge.
Boron on the periodic table 3. C Which structure is preferred. It should not be visible.
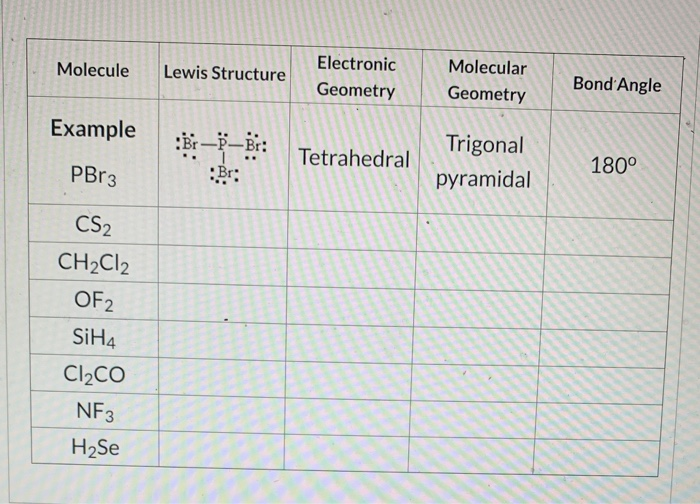
In b the nitrogen atom has a formal charge of 1. For example you would not like to have a structure with a positive formal charge on a highly electronegative element such as oxygen. Determine the Lewis structure of each of the following molecules.
Drawing PBr3 Lewis Structure is very easy to by using the following method. So the formal charge on Boron is 0. See the answer See the answer See the answer done loading.
A PBr3 b SiF4 c BF4. Feb 16 2021 Draw Lewis structure label formal charge C4H91 PBr3. со PBrg Select Draw Rings More Erase Select Draw Rings More Erase Q 91 eo 2 This problem has been solved.
6 valence electrons in isolated atom - 2 non-bonding electrons - ½ x 6 bonding electrons 6 - 2 - 3 1. Formal charge on an atom Number of valence electrons of the atom- number of electrons in its lone pairs- number of covalent bonds attached to the atom Hence satisfying the octet rule and. Phosphorus halides including phosphorus trichloride and phosphorus tribromide have been considered as potential replacements for Halon 1301 as fire suppressants.
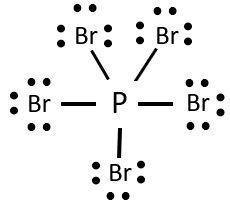
Formal charge on oxygen. This formula has a -2 formal charge on the outer N and 1 charges on the other two atoms. For Br the formal charge 7 052 6 0 holds true for all the three bromine atoms So now we put the single bonds to get our perfect Lewis Structure.
Include formal charges where appropriate. So the formal charge for this Bromine is 0 and since all these Bromines are the same the formal charge will be 0 for all of them. Likewise each bromine has three lone pairs 6 electrons and one electron in its bond to phosphorus.
Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule. In PBr3 you will find the phosphorus has two electrons from its lone pair and one electron in each bondThus phosphorus controls the five valence electrons it started with. Elementvalence electrons - of lone pairs 12 the electrons from each bond formal chargeP 5-041Br 7-6-10.
Minus bonding we said we have 6. Answer PBr5 Phosphorus pentabromide is Nonpolar What is polar and. What are the formal charges on PBr3.
Tribromide Br3- CID 77881 - structure chemical names physical and chemical properties classification patents literature biological activities safety. The formal charge on the nitrogen atom is therefore 5 - 2 62 0. Determine the Lewis structure of each of the following molecules.
Include electron lone pairs and any formal charges. 0 1 versus 1 2. Minus nonbonding theyre all in bonds so 0.
The formal charge on P is zero. Draw the Lewis Structure of CH3NCS including all resonance forms. The oxygen owns 2 non-bonding electrons and 3 bonding elections so the formal charge calculations becomes.
For P the formal charge 5 056 2 0. The phosphorus tribromide chemical formula is PBr3. PCl5 PF5 AsF5 PBr5 SbCl5 Linear.
Pbr3 molecular geometry learning about the lewis structure leads us to our next concept.

Pbr3 Lewis Structure Lewis Structure Of Pbr3 Phosphorus Tribromide Draw Lewis Structure For Pbr3 Youtube

Pbr3 Lewis Structure How To Draw The Lewis Structure For Pbr3 Youtube
Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity

Draw The Lewis Structure For Phosphorus Tribromide Pbr3 1 Clutch Prep

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist

6 2 Lewis Structures Introductory Chemistry

Video Lewis Dot Structure For Pbr3
Pbr5 Molecular Geometry Lewis Structure Shape Bond Angle And More

Pbr3 Lewis Structure How To Draw The Lewis Structure For Pbr3 Youtube

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist

Pbr3 Lewis Structure Phosphorus Tribromide Youtube

Draw The Lewis Structure For Phosphorus Tribromide Pbr3 1 Clutch Prep

What Is The Lewis Structure Of Pbr3 How Is It Determined Quora
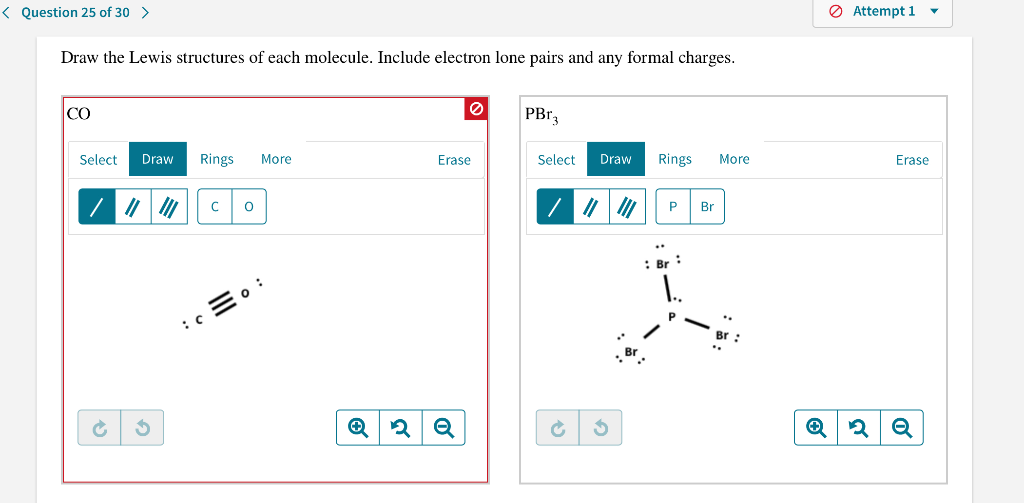
Question 25 Of 30 Attempt 1 Draw The Lewis Chegg Com

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist
Question 8 What Is The Formal Charge On The Br In The Chegg Com
Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity

Pbr3 Molecular Geometry Shape And Bond Angles Youtube