C2cl2 Lewis Dot Structure
Add one electron for each unit of negative charge. Determine the total number of valence electrons from all of the atoms in the molecule or ion.

C2cl4 Lewis Structure How To Draw The Lewis Structure For Tetrachloroethylene Youtube
Alternatively a dot method can be used to draw the CH 2 Cl 2 Lewis structure.

C2cl2 lewis dot structure. A step-by-step explanation of how to draw the CH2Cl2 Lewis Dot Structure DichloromethaneFor the CH2Cl2 structure use the periodic table to find the total. Carbon is less electronegative than Chlorine so itll go on the. Lewis Structure So42-Select The Atoms Drawn With Valid Lewis Dot Structures.
Lewis Structure Review How To Write Lewis Structures 1. Lewis dot Structure for CH2Cl2 generated from step-1 and step-2. For molecules of the formula ABn place the least electronegative element in the center.
Thus its Lewis structure must account for 22 valence electrons. The structures drawn using this theory are termed Lewis dot structures. Write the correct skeletal structure.
Cl-C Triple bond C-Cl. Because of this symmetrical geometry CCl 4 is non-polar. I apologize there isnt really a good way to show a triplebond.
Connect the exterior and core central atom of the CH2Cl2 molecule with four single bonds two C-Cl and C-H. In this stage use four single bonds to connect all two chlorine and two hydrogen atoms on the outside of the CH2Cl2 molecule to the central carbon atom in the middle. C2cl2 lewis structure.
Lewis Structure For Nh3. Lewis structures also known as lewis dot structures or electron dot structures are diagrams that represent the. Solved A 0 04336 G Sample Of Gas Occupies 10 0 Ml At 293 Chegg Com.
For the co lewis structure youll need a triple bond between the carbon and oxygen atoms in order to satisfy the octets of each atom while. Subtract one electron for each positive charge. Valence electrons inside a molecule.
What is the Lewis Structure for C2Cl2. Alternatively a dot method can be used to draw the lewis structure of COCl 2. Cl --- C Triple Bond C --- Cl.
Try drawing the Lewis structures of SO2 and SO32- a. Alternatively a dot method can be used to draw the lewis structure of COCl 2. How to Draw the Lewis Structure for.
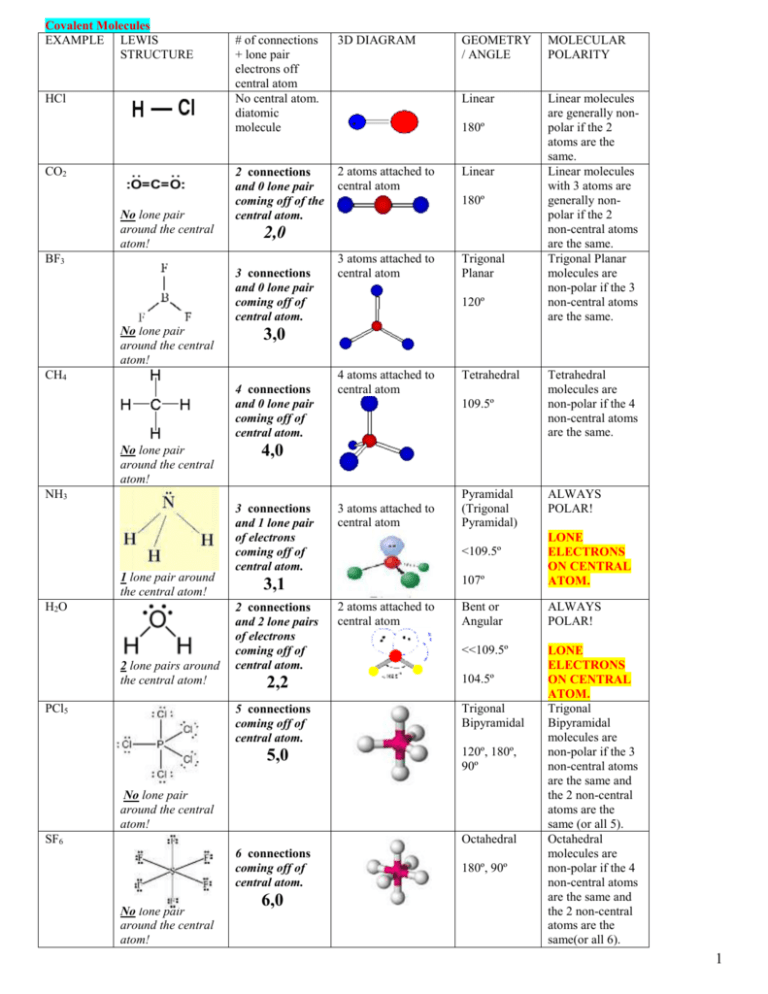
The molecular shape of the C2Cl2. Hydrogen however does tend towards a duplet not octet because it has only one electron in its K shell and thus. To understand it in detail we have to first get acquainted with the concept of Lewis Structure.
Lewis Structure is a 2D diagrammatic representation of the arrangement of electrons note. This molecule is made up of o. This brief flash video shows how atoms of calcium and chlorine react to form the salt calcium chlorideA step-by-step explanation of how to draw the CaCl2 Le.
This is reflected in the Lewis structure of the molecule. Hey GuysIn this video we are going to learn about the Lewis structure of CH2Cl2. A step-by-step explanation of how to draw the C2Cl6 Lewis Dot StructureFor the C2Cl6 Lewis structure calculate the total number of valence electrons for th.
A step-by-step explanation of how to draw the C2Cl2 Lewis Dot StructureFor the C2Cl2 Lewis structure calculate the total number of valence electrons for th. Count how many electrons from the outermost. SO32- acts as a Lewis base and SO2 acts as a Lewis acid.
The Lewis dot diagram for iodite IO2- is given below. COCl2 Lewis Structure. We will discuss the chemical bonding nature of phosgene in this article.
In the carbon tetrachloride molecule four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Using your VSEPR information determine the most likely molecular geometry shape for iodite. I think its similar to the Lewis structure for PCl5.
Since there is symmetry and no side has more electron density then the other the atom is non polar. Fcl Lewis Structure - C2Cl2 Lewis Structure. Please note that several atoms follow the octet rule ie they tend to achieve eight electrons in their valence shell through chemical bonding.
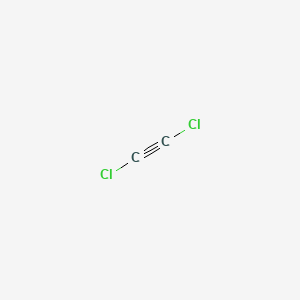
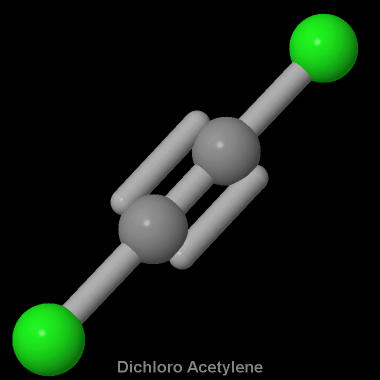
In the reaction CaOs SO2g --- CaSO3s Hint. Starting with its Lewis structure the C2Cl2 molecule has a total of 22 valence electrons 4 from each of the two carbon atoms and 7 from each of the two chlorine atoms. The molecular shape of the C2Cl2 molecule is linear.
Alternatively a dot method can be used to draw the CH 2 Cl 2 Lewis structure. A step-by-step explanation of how to draw the CaCl2 Lewis Dot StructureFor the CaCl2 Calcium Chloride Lewis structure calculate the total number of valen. It gives us a graphical sketch with electron-dot notations for us to.
Lewis Dot of Carbon TetraChloride CCl4 TetraChloromethane. Ca2 acts as a Lewis base and SO32- acts as a Lewis acid. What is the Lewis dot structure of CCl4.
It is a chemical formula for Dichloromethane.
What Is The Molecular Shape Of C2cl2 Socratic

Video Lewis Dot Structure For Pbr3

Chapter 6 The Shape Of Molecules Ppt Download
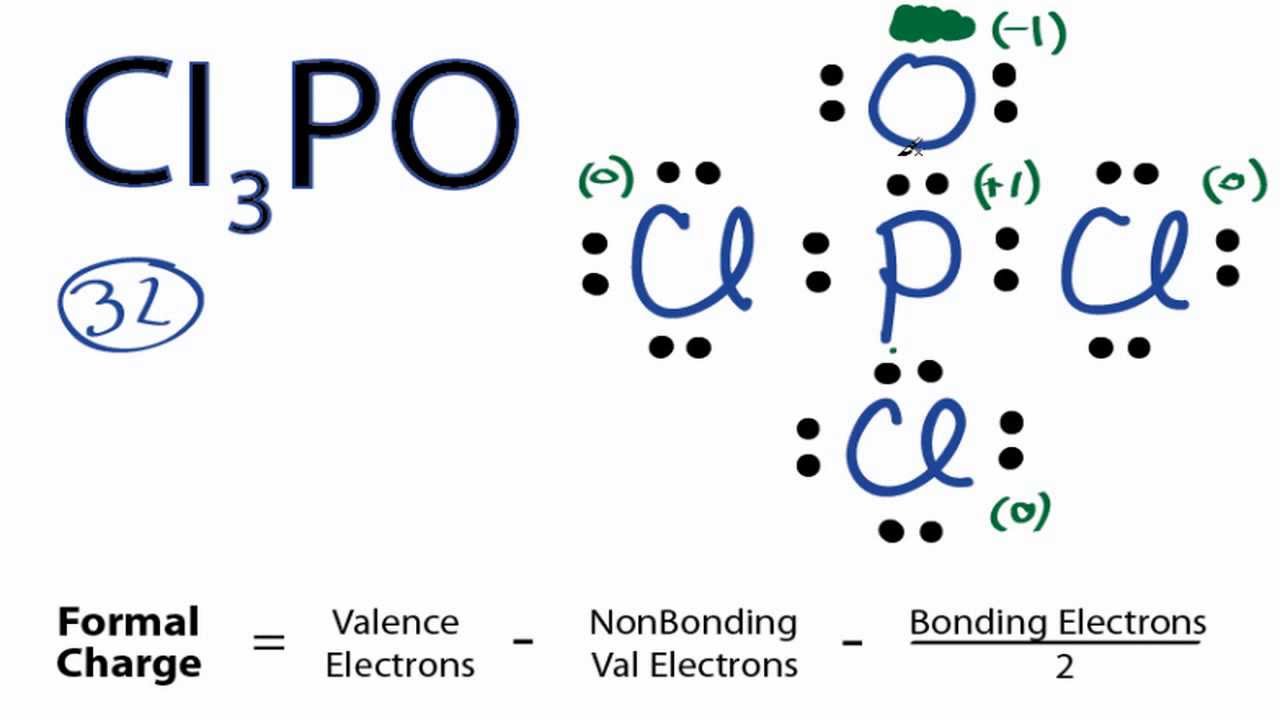
Cl3po Lewis Structure How To Draw The Lewis Structure For Cl3po Youtube
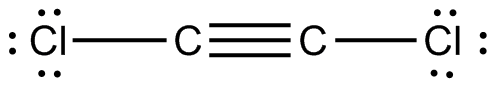
What Is The Molecular Shape Of C2cl2 Socratic

What Is The Molecular Shape Of C2cl2

Draw A Lewis Structure For C2cl2 And Indicate How Many And What Types Of Bonds Are Present Brainly Com

C2cl4 Lewis Structure How To Draw The Lewis Structure For Tetrachloroethylene Youtube
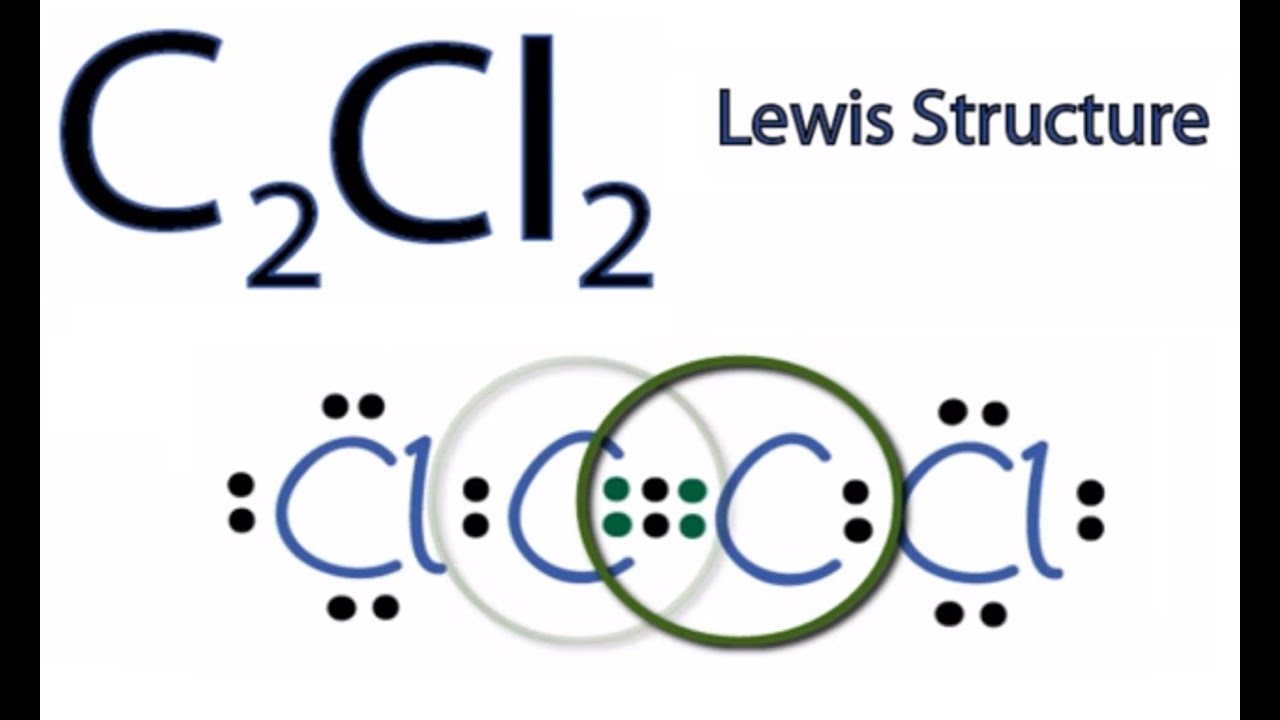
C2cl2 Lewis Structure How To Draw The Lewis Structure For C2cl2 Youtube

Video Lewis Dot Structure For Pbr3

Draw A Lewis Structure For C2cl2 And Indicate How Many And What Types Of Bonds Are Present Brainly Com

Lewis Vsepr And Contour Diagrams For C2cl2 Youtube
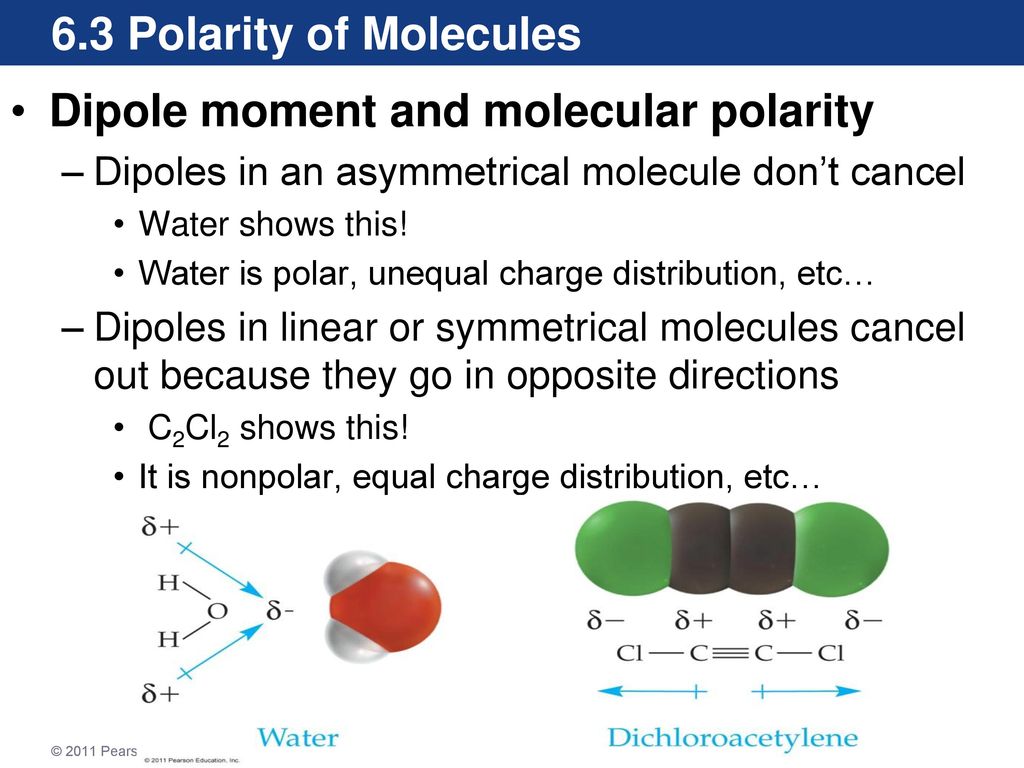
Chapter 6 The Shape Of Molecules Ppt Download

Determine The Hybridization Of The Following Molecules A C2cl2 B Co2 C O3 D H2o Study Com
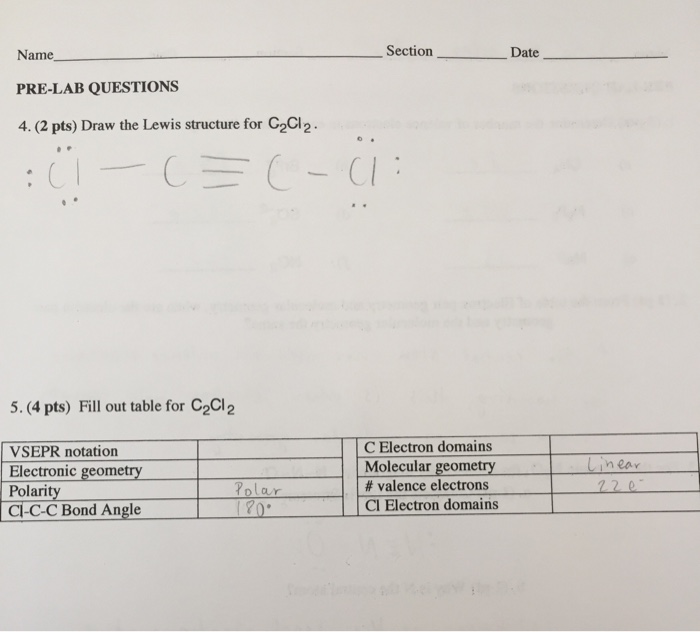
Section Date Name Pre Lab Questions 4 2 Pts Draw Chegg Com

Video Lewis Dot Structure For Pbr3
Dichloroacetylene C2cl2 Pubchem