C2h4 Lewis Diagram
Lewis Dot Structure for C2H4 6 of 6 Watch the video of Dr. Step method to draw lewis structure of ethylene Ethene.

A Compound X Is C2h4 Draw Its Electron Dot Structure Will It Dissolve In Water Brainly In
Hutchinson 2009-09-01 The Practice of Chemistry-Donald J.

C2h4 lewis diagram. 2 Draw theThe Lewis dot diagram of CH2CHCN should be simple to draw from thisstructural formula. Lewis Structure Hybridization. The general rules for drawing electron dot diagrams are as follows.
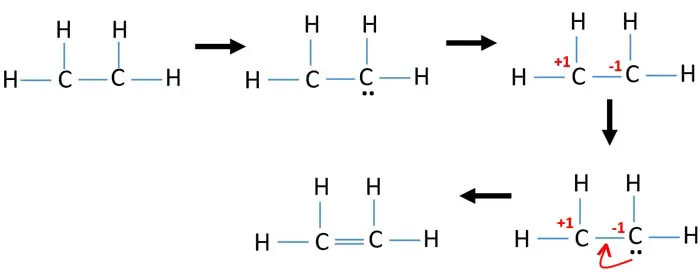
Note that the C 2 H 4 Lewis dot structure involves sharing more than one pair of electrons. We have 12 available valence electrons. Draw the Lewis structure for C2H4.
Lewis Structures for C2H4. Alternatively a dot method can be used to draw the lewis structure. 1 Draw electron dot diagrams for each of the atoms present.
Follow three steps to find C2H4 molecular geometry 1. We place two valence electrons between each atom as shown in the figure. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
Find the Number of lone pairs present on the central atom of the C2H4 Lewis structure. 1 point for the correct selections assessed when you answer and 5 points for the Lewis structure on your work assessed when I review. Is C2H6 planar or nonplanar.
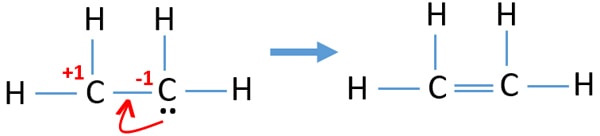
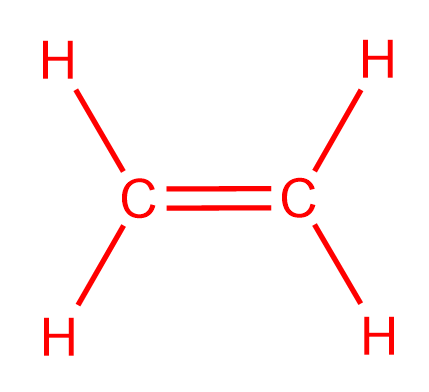
If you look at the Lewis structure for C2H4 it appears to be a symmetrical molecule. Ethylene C2H4 has the Lewis Structure. In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
Lewis dot structure of C 2 H 4. Drawing the Lewis dot structure for C2H4 ethene and answer the questions below. This means that the carbon atoms share 4 electrons.
C2H4 Lewiss dot structure is very helpful to find Its molecular geometry because the lewis diagram helps us to determine how many bond pairs and lone pairs a molecule contains. The molecular shape is predicted to be trigonal planar around each carbon atom. Is C2H4 planar.
This is ethane an alkyne double H to H with 2 carbon atoms which means that the relationship between the carbon atoms is double. Step-by-step tutorial for drawing the Lewis Structure for C2H4Drawing the Lewis Structure for C 2 H 4. Each carbon atom will have the shape of a flat triangle.
C2H4 Lewis Structure. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. Start by forming covalent bonds between the Carbon and Hydrogen atoms.
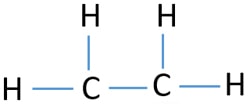
However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure. For C 2 H 4 you have a total of 12 total valence electrons. Hydrogen is the least electronegative element here.
Put two carbons in the center and arrange hydrogena toms on the sidesArrange electrons until both carbons get 8 electrons. Lewis Structures for C2H4. Electron Dot Structure for ethane C2H4.
This is composed of a σ framework and a π-bond. Calculate the total valence electrons in the molecule. The ethylene is a covalent bond and lewis base.
Step-by-step tutorial for drawing the Lewis Structure for C2H4. Lewis Structures for C2H4. This means that the carbon atoms have 120 degrees between SP2 hybridization and bonds.
For C 2 H 4 you have a total of 12 total valence electrons. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial. Lets see how to find this.
Hybridization of atoms in ethene molecue can be found from lewis structure. How_to_do_lewis_dot_structure_for_c2h4 35 How To Do Lewis Dot Structure For C2h4 that respond to key market needs for detailed and modern treatment of organic chemistry embracing the power of visual learning and conquering the challenges of effective problem solving and assessment. It is a chemical formula for Ethylene or Ethene.
Most stable structure is taken as the lewis structure of ethene. Arrangement of atoms shown below dashed lines show connections between atoms. H-----C---H SHOW WORK This question is worth a total of 6 points.
C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule. However to determine if C2H4 is polar we consider the molecular geomet. The molecular geometry of C2H4 is trigonal planar above are the explanation of its Lewis structure.
Step-by-step tutorial for drawing the Lewis Structure for C2H4. Ethenes lewis structure can be built by VSEPR rule. How_to_do_lewis_dot_structure_for_c2h4 24 How To Do Lewis Dot Structure For C2h4 Books How To Do Lewis Dot Structure For C2h4 Concept Development Studies in Chemistry-John S.
Ethylene C2H4 has sp2 hybridization and its bond angle is between 1166 to 1217. Lewis structure of C2H4. Alternatively a dot method can be used to draw the lewis structure.
Lewis structure of C2h4.

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Hybridization Structure Of Ethylene Mcc Organic Chemistry
Ethene C2h4 Lewis Structure Hybridization

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure C2h4 Lewis Structure Molecular Geometry

C2h4 Molecular Geometry Shape And Bond Angles Youtube

Structure And Bonding In Ethene The Pi Bond Chemistry Libretexts
Lewis Electron Dot Structures Ck 12 Foundation

Draw The Lewis Structure For The C2h4 Ske Clutch Prep

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In
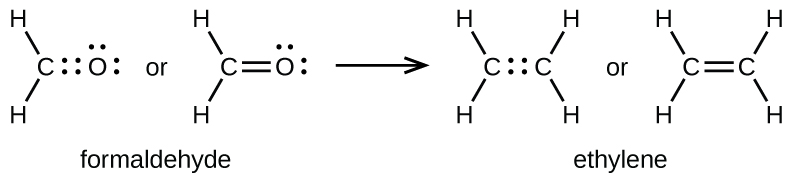
7 3 Lewis Symbols And Structures Chemistry
Draw The Lewis Structure For The C2h4 Ske Clutch Prep
Ethene C2h4 Lewis Structure Hybridization
Lewis Structure Of C2h4 Biochemhelp
Ethene C2h4 Lewis Structure Hybridization
