Lewis Structure Of Hcn
It is also called prussic acid. HCN Lewis Structure Molecular Geometry Hybridization MO Diagram and Polarity Hydrogen Cyanide is a very toxic acid and is famous for causing irritation in the eyes and respiratory system if any human inhales HCN in substantial quantity.
Hcn Lewis Structure Molecular Geometry Shape And Polarity
The associated charge balances the 7 protons in the nitrogen nucleus so the nitrogen is formally neutral.

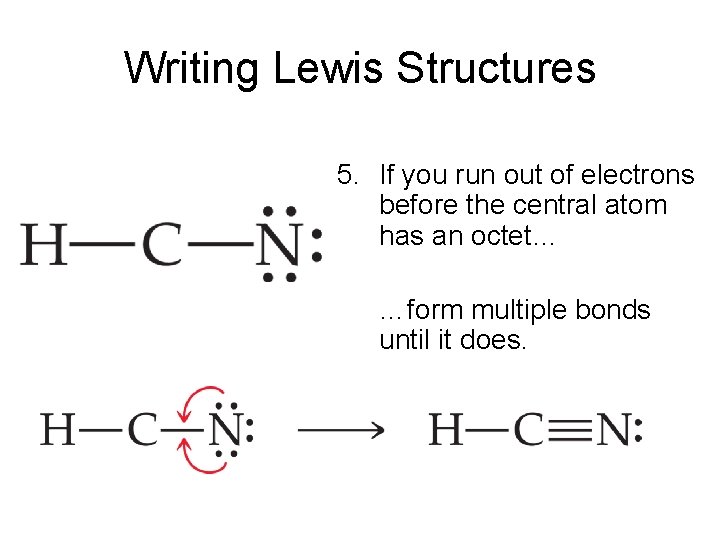
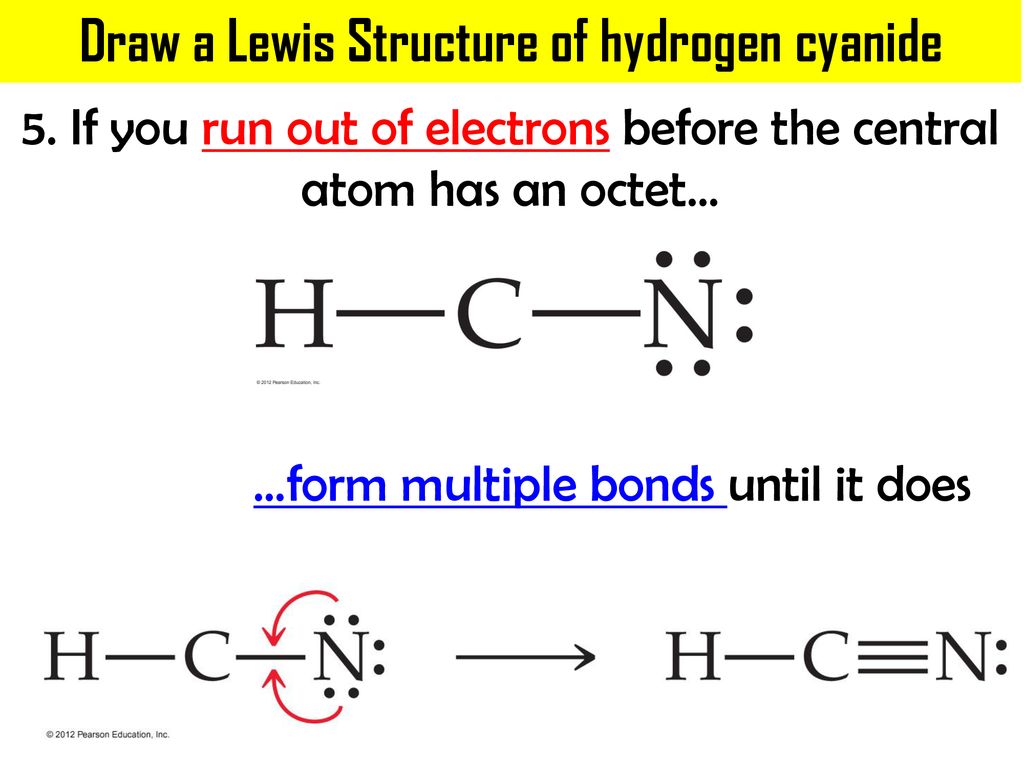
Lewis structure of hcn. Arrange electrons until both carbon and nitrogen get a triple bond giving an octet and hydrogen has 2. HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element. Then bond the carbon atom to a single hydrogen atom.
4 rows HCN Lewis structure comprises three different atoms. Draw the skeletal structure showing how the atoms are connected using single bonds. Explain the configuration in the molecule of valence electrons around the atoms.
Hydrogen has one bond and no lone pairs. Once you have arranged the atoms start placing the valence electrons around. A chemical compound with the formula HCN is hydrogen cyanide.
Lewis structure shows how atoms are bonded with each other. It is a liquid. TranscriptFor the HCN Lewis structure we have one valence electron for Hydrogen we have four for Carbon and we have five for Nitrogen for a total of ten valence electrons for the HCN Lewis structure.
The Lewis structure for HCN otherwise known as hydrogen cyanide is fairly simple. Five electron pairs as required. Draw the Lewis Structure of HCN.
As Carbon is the least electronegative atom in this molecule it will take the central position. Hydrogen cyanide is a one- carbon compound consisting of a methine group triple bonded to a nitrogen atom It has a role as a human metabolite an Escherichia coli metabolite and a poison. D In the most favorable Lewis structure for HCN there is 1 single bond 1 triple bond and 1 lone pair of electrons.
Chemistry questions and answers. E In the most favorable Lewis structure for CO there is 1 double bond and 3 lone pairs of electrons. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this.
It is a. Draw The Lewis Structure For HCN. It is a conjugate acid of a cyanide.
Draw the Lewis structure of HCN and then determine its electron domain and molecular geometries. The compound is a colorless substance that is available in liquid or gaseous form. Well put the Carbon in the center because its less electronegative than the Nitrogen and Hydrogens always go on the outside of Lewis structures.
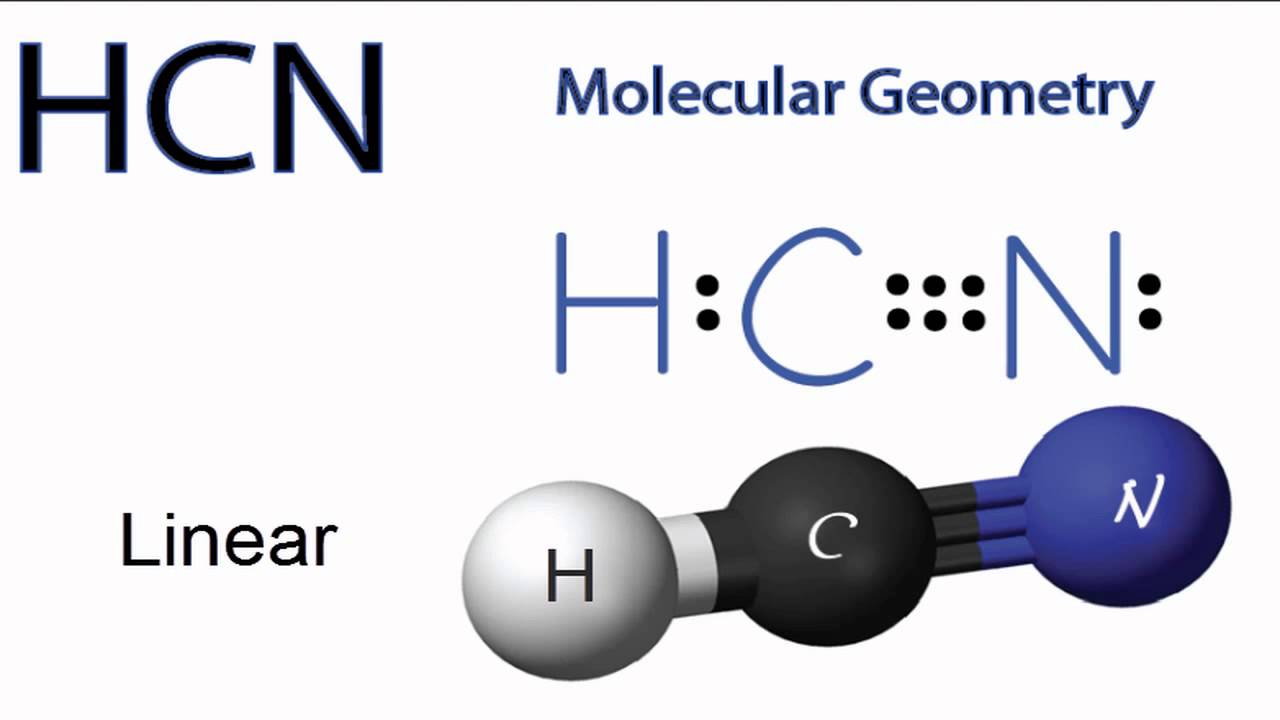
Usually try to draw the most symmetrical structure with the atom of least electronegativity in the center. Place the carbon atom in the center and triple bond it to a nitrogen atom. H C N 180.
The carbon atom has or shares 3 electrons from the triple bond and a lone pair of electrons which it owns. And then place the remaining atoms in the structure. Draw the Lewis structure Molecular geometry for HCN.
To start with making the Lewis Structure of HCN we will first determine the central atom. A tetrahedral planar B trigonal planar tetrahedral C tetrahedral bent 1095 D linear linear E bent 120 bent 1095 Click to draw a new structure. In this case you cant really draw a symmetrical structure.
It is a hydracid and a one- carbon compound. With 2 inner core electrons this makes 7 electrons with which. For the HCN Lewis structure calculate the total number of valence electrons for the HCN molecule.
The nitrogen atom will have a lone pair placed on it. After determining how many valence electrons there are. Draw the Lewis structure for eqtext HCN eq.
Lewis structure of HCN Uncharged nitrogen has three bonds and one lone pair. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom. It is based on the fact that the representative elements acquire a.
Draw The Lewis Structure For HCN. Draw The Lewis Structure For HCN. D In the most favorable Lewis structure for HCN there is 1 single bond 1 triple bond and 1 lone pair of electrons.
Hydrogen carbon and nitrogen. And thus H C N. Put carbon in the center and arrange hydrogen and nitrogen atoms on the sides.
The nitrogen nucleus has 3 electrons from the triple bond and 2 electrons from its lone pair and 2 inner core electrons.

How To Determine The Lewis Structure For Hydrogen Cyanide Quora
Hcn Molecular Geometry Youtube

Hcn Lewis Structure Hydrogen Cyanide Youtube
Lewis Structures Chapter 8 Lewis Structures Lewis Structures

Draw The Lewis Structure For Hcn Molecule Clutch Prep

Hcn Lewis Structure How To Draw The Lewis Structure For Hcn Youtube
Answer In Organic Chemistry For Tj 92397

Draw The Lewis Structure Of The Following Clutch Prep
![]()
Draw Lewis Structures For The Following Compounds And Determine The Shape For Each Molecule A Hcn B So3 C Nh4 D Sncl4 Study Com
6 4 Lewis Structure Diagrams Ppt Download

Hcn Lewis Structure Molecular Geometry What S Insight

Draw The Lewis Structure For The Hydrogen Cyanide Molecule Hcn C Is The Center Atom Study Com

Hcn Lewis Structure Molecular Geometry Hybridization Mo Diagram And Polarity Techiescientist

Hcn Lewis Structure How To Draw The Lewis Structure For Hcn Youtube

Hcn Lewis Structure Hydrogen Cyanide Youtube

Lewis Structure And Hybridization Of Hcn Hydrocyanic Acid Hydrogen Cyanide Lewis Structures

Hcn Lewis Structure Molecular Geometry Hybridization Mo Diagram And Polarity Techiescientist

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Makethebrainhappy The Lewis Dot Structure For Hcn