Sif4 Lewis Structure Bond Angle
Write the bond lengths and bond angles in each case. Explain Why Lewis Dot Structure For S2Cl2 IS Cl - S - S - Cl Why Does The Two Sulfurs Have To Be Beside Each Other And The Two Chlorines Have To Bethe First And LastWhat Is The Lewis Dot Structure Of S2Cl2.

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
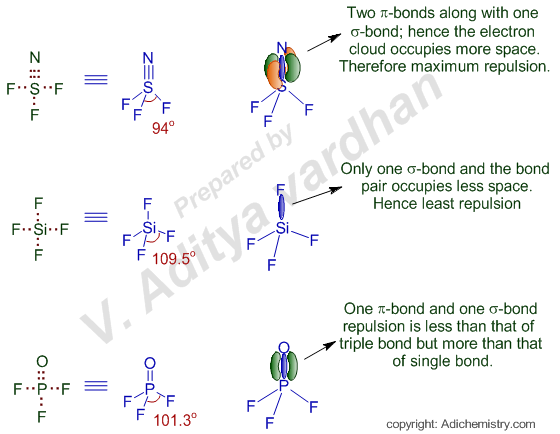
Hence the bond angle will be medium in this case.

Sif4 lewis structure bond angle. What hybridization is suggested by the bond length and angles in methane. In the tetrahedral geometry of silicon tetrafluoride two Si-F bonds are in a plane one Si-F bond is above the plane and another Si-F bond is present below the plane. This means that SeF4 has Trigonal Bipyramidal structure with 4.
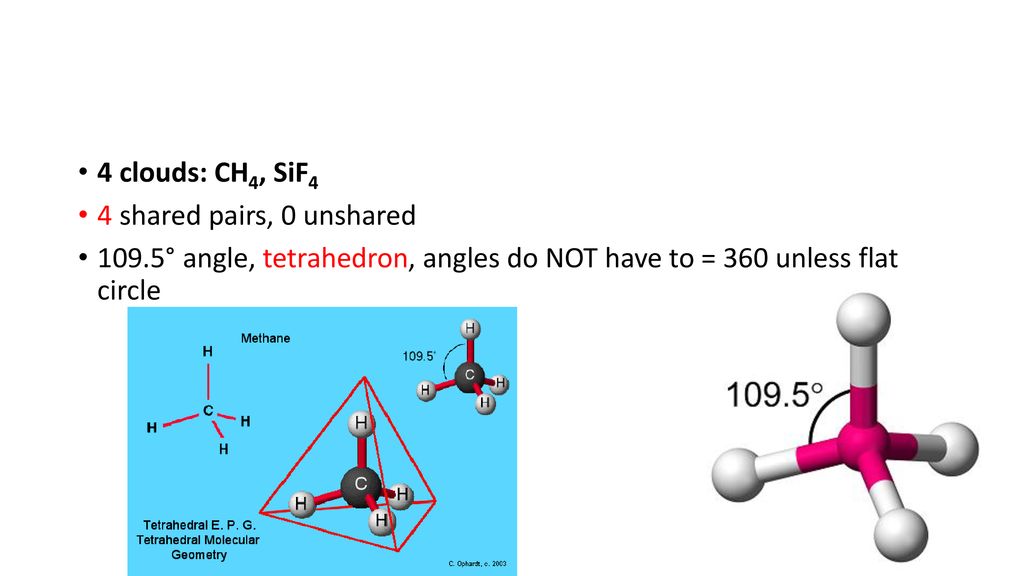
The electron geometry for. People also ask is SiF4 polar or nonpolar. The tetrahedral geometry of silicon tetrafluoride leads to the F-Si-F bond angle of 1095.
Lewis structure for SeO3. Due to four negative charge centresfour electron pairsfour electron domains two of which are lone pairstetrahedral arrangement of electron pairs. Carbon on the left AB 4 tetrahedral bond angles 1095 carbon in center AB 3 trigonal planar bond angles 120 oxygen on right AB 2 E 2 bent bond angle.
Explain Why SiF4 Has A Bond Angle Of 1095 Degree2. The bond angle is least affected in case of SiF 4 since all the Si-F bonds are single bonds which exert less repulsion on other bond pairs. These are arranged in a trigonal bipyramidal shape with 102 F-S-F bond angles between the equatorial fluorine atoms and 173 between the axial fluorine atoms.
Writing electron configurations for. Sif4 bond angle. Then what is the molecular geometry of SeF4.
Remove s electrons first then d electrons. Very toxic by inhalation. Normally the axial F-S-F angle would be 180 because of course they are exactly oppositeantiparallel to each other but it becomes 1731 and normally the equatorial F-S-F angles are 120 because the horizontal plane would distribute three connected atoms evenly over 360 but they become 1016.
An explanation of the molecular geometry for the SiF4 ion Silicon tetrafluoride including a description of the SiF4 bond angles. Under prolonged exposure to heat the containers may rupture violently and rocket. Vapor is heavier than air.
Click to see full answer. Lewis structure for PFCl2. Allow any bond angle in the range 97 to less than 1095 experimental value is 98.
SiF4 Dot Lewis Structure Molecular Geometry Bond Angle Polar or Nonpolar. Response times vary by subject and question complexity. 90 And 180 All Are 90 All Are 1095 90 And 1095 2 10 2 1 Point Draw Lewis Structures the Central Atom Is Underlined In Order To Rank The Species In Order Of Increasing Bond Angle.
Lewis Structure For Sicl4 SiCl4 Lewis Structure How to Draw the Lewis Structure SCl4 Lewis Structure. Lewis Structure For Sicl4. Hence the bond angle is maximum ie.
This will reduce the bond angle more than in other cases. The axial Se-F bonds are 177 pm with an F-Se-F bond angle of 1692. Quiz your students on SiF4 Dot Lewis Structure Molecular Geometry Bond Angle Polar or Nonpolar using our fun classroom quiz game Quizalize and personalize your teaching.
The reason is that the lone pair prefers one of the equatorial positions. Lewis structure for IF5. Bond angle octehedral drawn square planar 90 180.
3-D structure MG approx. Lewis structure for CN-draw it. Explain the differences and similarities.
SF4 Bond angles and shape The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. Fluorine atoms on the equatorial positions have the bond angles of 102 degrees and the axial ones have 173 degrees which are a little different than the trigonal bipyramidal molecular geometry leading to a see-saw shape. The bond length of the Si-F bond.
A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure Silicon TetrafluorideFor the SiH4 structure use the periodic table to find the tota. Quiz your students on Lewis Structure For BI3 Molecular Geometry Bond Angle Hybridization Polar or Nonpolar using our fun classroom quiz game Quizalize and personalize your teaching. Thereof what is the molecular geometry of SeF4.
How to Draw the Lewis Structure for El enlace químico i SiH4 Lewis Structure Molecular Geometry YouTube. Silicon tetrafluoride appears as a colorless nonflammable corrosive and toxic gas with a pungent odor similar to that of hydrochloric acid. Silicon Tetrafluoride SiF4 Lewis Dot Structure YouTube.
November 28 2020 Articles. Draw Lewis-dot structures molecular geometries for XeF4 SF4 SiF4 CF4 and SnF4. The Shapes Of Molecules.
In POF 3 there is a double bond between P and O which also causes more repulsion than single bond but less than the triple bond. Ideal ax-ax 180 eq-eq 120 a. Bond angle s.
SiF4 is not polar as the fluorines negative dipoles cancel each other out as the are all pulling away form the centre equally the centre being silicon which has a lower electronegativity than fluorine. Draw Lewis-dot structure molecular geometry for methane. Lewis structure for KrF4.
What Is The Molecular Geometry Of Sif4 Quora
Vsepr Theory Bond Angles Nsf3 Sif4 Pof3
7 10 Notes Shapes For Covalent Structures Ppt Download
1 1 Point Draw Lewis Structures The Central Atom Is Chegg Com

For Each Of The Molecules Below Determine The Chegg Com
Chem Molecular Shape Molecular Geometry Scientific Tutor

Sif4 Molecular Geometry Bond Angles Electron Geometry Youtube
Chem Molecular Shape Molecular Geometry Scientific Tutor

Silicon Tetrafluoride Sif4 Lewis Dot Structure Youtube

Determine The Electron Geometry Eg And Molecular Geometry Clutch Prep

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Sif4 Lewis Structure How To Draw The Dot Structure For Sif4 Youtube

What Is The Shape Of Sf4 Molecule Quora

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist



