H2so4 Is Lewis Acid
In other words a Lewis acid is an electron-pair acceptor. H2so4 is a chemical formula of sulfuric acid which is commonly known as oil of vitriol.

Non Aqueous Solvents Nh3 Hf H2so4 N2o4 Pocl3 Socl2 Brf3 In 2021 Solvents Chemistry Agno
Sulfuric acid can supply up to 2 H ions per molecule and H ions are Lewis Acids.

H2so4 is lewis acid. Properties of HSO4-Sodium Bisulfate NaHS04 which is the pure substance starts melting at 185C and begins to lose water and forms the hydrogen sulphate ion HSO4. Total valence electrons concept is used to draw the lewis structure of H 2 SO 4. A Lewis base is any substance such as the OH- ion that can donate a pair of nonbonding electrons.
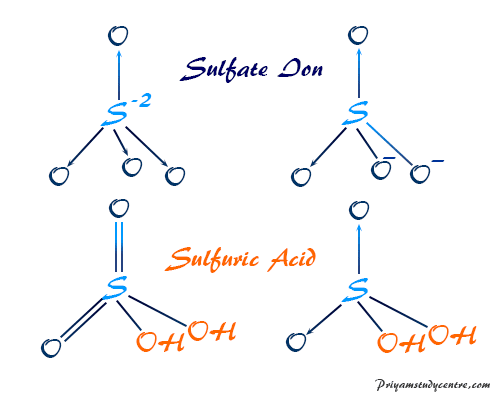
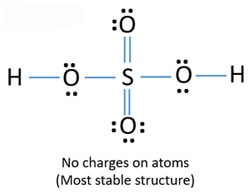
This means that the hydrogen atoms will be attached to. In H3O ion there is no vacant orbital octet of oxygen is fulfilled and duplet of hydrogen atoms are also fulfilled. Therefore there should be two -OH groups in H2SO4 molecule.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features. Sulfuric acid is a dibasic strong acid. Schwefelsäure ist eine chemische Verbindung des Schwefels mit der Summenformel H 2 SO 4Sie ist eine farblose ölige sehr viskose und hygroskopische FlüssigkeitSchwefelsäure ist eine der stärksten Säuren und wirkt stark ätzendDiese Mineralsäure bildet zwei Reihen von Salzen die Hydrogensulfate und die Sulfate bei denen im Vergleich zur freien Säure ein beziehungsweise zwei.
Answer verified by Toppr Upvote 0. H2So4 is a lewis acid because it is a bronsted acid. Its a mineral acid composed of elements like oxygen hydrogen and sulfur.
Farblose ölige Flüssigkeit 100 Teflonverschluss konzentrierte Säure oder konzentrierte Lösungen Gefahrenklassen Kategorie Ätz-Reizwirkung auf die Haut. Because sulfuric acid is a dibasic acid it can release two H ions in the water. A step-by-step explanation of how to draw the H2SO3 Lewis Structure Sulfurous acid.
This is the h2so4 lewis structure. H2So4 is a lewis acid because it is a bronsted acid. Chem 1090 Lewis 8b A Lewis structure for the oxyacid sulfuric acid.
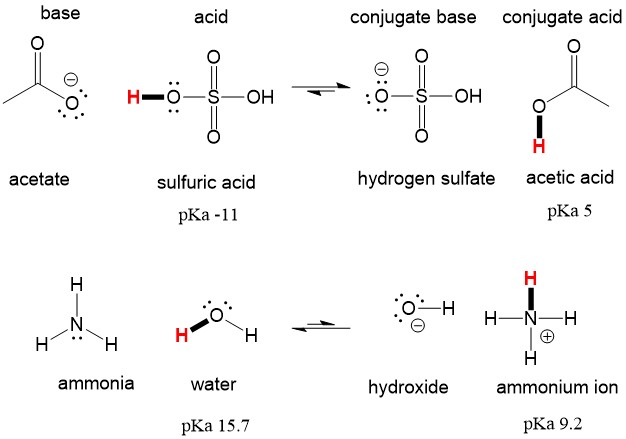
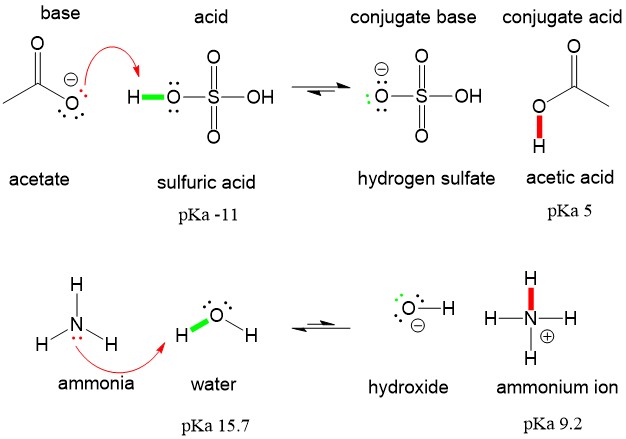
If it were able to act as a base then you should get H3SO4 as product. Although the hydronium ion is the nominal Lewis acid here it does not itself accept an electron pair but acts merely as the source of the proton that coordinates with the Lewis base. A Lewis acid is any substance such as the H ion that can accept a pair of nonbonding electrons.
H2SO4 is not amphoteric because it can not act as a base. Is H2SO4 Lewis acid. The answer is D.
It means it can release two hydrogen atoms to show acidic characteristics. It has a molecular weight of 98079 g mol. A Lewis base is therefore an electron-pair donor.
No H3O is not a Lewis acid. ALL bronsted acids are lewis acids. To be a Lewis acid the molecule or ion should possess a vacant orbital or a π-bond in which it can accept a lone electron pair.
Therefore we can assume there should be two -OH bonds in sulfuric acid molecule. The structure is similar to that of methane. For H2SO4 molecule sulfur has the highest valence than oxygen and hydrogen.
Molmasse AGW pK s-Wert H 2 SO 4 pK s-Wert HSO 4 Dichte Schmelzpunkt Siedepunkt Wasserlöslichkeit. Transcribed image text from this question. H2SO4 Lewis Structure Molecular Geometry and Hybridization H2SO4 is a chemical formula of Sulfuric acid which is commonly known as Oil of Vitriol.
With two bonding pairs and two lone pairs the oxygen atom has now completed its explain why the following lewis. The point about the electron-pair remaining on the donor species is especially important to bear in mind. ALL bronsted acids are lewis acids but not the other way around.
When we have an H or H2 in front of a polyatomic molecule like CO. An experimental investigation was conducted to understand the roles of the Brønsted acid H 2 SO 4 and Lewis acid Al 2 SO 4 3 in methyl levulinate ML production from biomass carbohydrates including glucose fructose and cellulose. Are Lewis acids whereas H 2 O is a lewis base.
Its a mineral acid composed of elements like oxygen hydrogen and sulfur. Sulfuric acid is a strong dibasic acid. 98079 gmol 01 mgm 3 TRGS 900 3 199 184 gcm 3 1031 C 337 C in jedem Verhältnis mischbar.
It has a molecular weight of 98079 gmol. Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing When we have an h or h2 in front of a polyatomic molecule like co3. Sulfuric acid H 2 SO 4.
The key to. H2So4 Lewis. The answer is D.
Because according to Lewis concept of acid-base lone electron pair acceptors are Lewis acids. Hence this is the most stable Lewis structure for HSO4-. En este vídeo comento los pasos necesarios para escribir la estructura de lewis del ácido sulfúrico h_2so_4.
H2SO4 works as an oxidizing and dehydrating agent. H2so4 lewis structure molecular geometry and hybridization. It is classified as a weak acid as it partially dissociates to form H and SO42-.
H2so4 works as an oxidizing and dehydrating agent.

Dissolution Mechanism Of Cellulose In 72 Wt Sulfuric Acid Based On Download Scientific Diagram

How To Calculate The Formal Charges For H2so4 Sulfuric Acid Youtube

Pin On Chemistry What Is Chemistry

H2so4 Lewis Structure Sulfuric Acid Youtube

A Reaction Map Pdf For Benzene And Aromatic Compounds Organic Chemistry Reactions Organic Chemistry Reactions

Safety Data Sheet Nitrosyl Sulfuric Acid

My Friend Tried To Find The Structure For Sulphuric Acid On The Internet Cursed Chemistry
Sulfuric Acid H2so4 Structure Production Properties Uses
Sulfuric Acid H2o4s Chemspider
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing
Reviewing Acid Base Definitions Organic Chemistry Help
Reviewing Acid Base Definitions Organic Chemistry Help

Dissolution Mechanism Of Cellulose In 72 Wt Sulfuric Acid Based On Download Scientific Diagram

Tetryonics 56 04 Common Acids Are Formed Where Protons Bind To Chemical Elements And Compounds To Create Incomplete Atomic Orbitals And Charged Cations

File Sulfuric Acid Lewis Png Wikimedia Commons



