Does So3 Follow The Octet Rule
The octet rule is a helpful guide to understanding nitrogen chemistry. Heavier elements and transition metals violate the octet rule much more often than lighter elements such as.

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
This is due to the presence of d orbitals in sulfur since its in the third period and can accommodate up to 18 electrons in its outermost shellthough its less favourable to form more bonds and receive electrons after achieving octet configuration.

Does so3 follow the octet rule. Does NO2 follow octet rule. Do not obey octet rule. Sulfur violate the octet rule.
Although they are few. There are three violations to the octet rule. We can say that it follws extended form octet rule.
Simply we can see in the structure or SO2 that oxygen1 has 8 e in outermost shell as 2 it shares with S. Subsequently question is is so3 2 a resonance structure. True or false.
Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. ChemDoodle What is the electron-pair geometry for S in SO3. Nitrogen dioxide is the chemical compound with the formula NO2.
Elements classified as metals tend to. Sulfur can expand its octet and can accommodate up to 12 electrons because of which it does not follow the octet rule. Odd-electron molecules A molecule with an odd number of electrons in the valence shell of an atom.
Nitrogen generally follows the octet rule. Wikipedia has a page on hypervalency and from an answer here. However it is hard to imagine that one rule.
Having an odd number of electrons in a molecule guarantees that it does not follow the octet rule because the rule requires eight electrons or two for hydrogen around each atom. Does NOCL follow the octet rule. Does NF3 follow the octet rule.
I hope you guys are clear regarding the Lewis structure of SO3. Sulfur forms an expanded octet. Does N2O violates the octet rule.
Sulfur is a 3rd-period element. As there is a small energy difference between 3p and 3d shells with the help of a little excitation an unpaired electron can move from the 3p shell to the 3d shell easily. The total number of valence electrons is 52617.
Also which elements can violate the octet rule. It has less than an octet on at least one atom. As with many rules there are exceptions or violations.
Sulfur can do so as it has access to energetic 3d-subshell for bonding. There are seven resonance structures for SO_3. More SO3 will be produced Kc will increase.
70 More Lewis Dot Structures. A dative bond is formed and so it also get 8 e in outermost shell. The octet rule states that elements gain or lose electrons to attain an electron configuration of the nearest noble gas.
Oxygen2 does not share instead is donated 2e by S ie. I SO3 ii SO32- iii NO3- iv PF3 v BF3 only i and ii all are planar except iv. Sulfur having valence electrons in the 3rd energy level would also have access to the 3d sublevel thus allowing for more than 8 electrons.
Describe the formation of an ion from a metal and a nonmetal in terms of the octect rule. NOT IN THIS MOLECULE. BH3 doesnt follow octet rules but B2H6 is.
Answered 3 years ago Author has 64 answers and 461K answer views. In S F 4 the central S atom has in all 10 electrons around it while each F atom has eight. Again nitrogen dioxide does not follow the octet rule for one of its atoms namely nitrogen.
Hence it can use its 3d orbitals to make more than 4 bonds. Do not include overall ion charges or formal. In order to do that we will calculate the total number of valence electrons in each of the following and then write the Lewis structure.
Which of the following substance isare planar. Typically elements in the first two rows of the periodic table form compounds in accordance with the octet rule. Three cases can be constructed that do not follow the Octet Rule and as such they are known as the exceptions to the Octet Rule.
N2O CS2 PH3 CCl4 NO2. Do not dra w double bonds to oxygen atoms unless they are needed for the central atom to obey the octet rule. There are some notable nitrogen-based molecules that violate this rule.
Now coming to S it has 6e of its own and 2 e which it. And the reason is that all formal charges become 0. This does not mean that the octet rule is uselessquite the contrary.
Draw t the Lewis structure for SOs in the window below and then answer the questions that follow. It will hold more than 8 electrons. When you draw the Lewis structure you first get the three structures at the top.
In the given problem we are going to find out in which of the given molecules or ions the central atom does obey the octet rule. How does Mg obey the octet rule. So if you recalculate the things at last it will be like 6 0 6 0.
In each of them S has a formal charge of 2 and two of the O atoms have formal charges of -1. That means that it doesnt really obey the octet rule allowing it to take on extra electrons. Represent the first violation to the octet rule.
SO32- has 3 resonance structures one for each structure that is formed when sulfur makes a double bond with oxygen. The most commonly encountered stable species that exist with an odd number of electrons are nitrogen oxides such as nitric oxide NO and nitrogen dioxide NO 2 both of which are free radicals and disobey the octet rule. S does not follow the octet rule.
The octet rule is a chemical rule of thumb that reflects the observation that elements tend to bond in such a way that each atom has eight electrons in its valence shell giving it the same electronic configuration as a noble gas. In the bottom structure all atoms. AlH3 doesnt follow octet rules but Al2H6 is.
Only N2O3 molecule follow octet rule. What is the the shape. So3 doesnt follow octet rule because it forms three double bonds with oxygen atoms and thushas 6 6obtained from oxygen atoms12 electrons.
How does N obey the octet rule. In each of the three structures in the middle S has a formal charge of 1 and one of the O atoms has a formal charge of -1. Which molecule has a Lewis structure that does not obey the octet rule.
BF3 can best be described through the lewis structure as.

Calculating So3 Formal Charges Calculating Formal Charges For So3 Youtube
What Are All Resonance Structures For So3 Socratic

How To Determine The Lewis Dot Structure Of So3 Quora

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

So3 Lewis Structure How To Draw The Lewis Structure For So3 Sulfur Trioxide Youtube
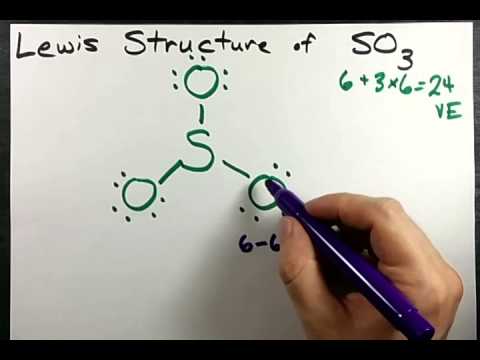
Lewis Structure Of So3 Sulfur Trioxide Youtube

Why So3 Does Not Follow Octet Rule S Could Simply Form A Double Bond With Any One Of The Oxygen Atom Quora
Why So3 Does Not Follow Octet Rule S Could Simply Form A Double Bond With Any One Of The Oxygen Atom Quora
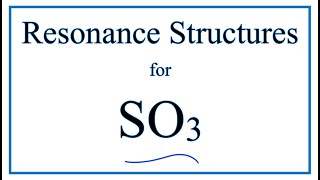
Resonance Structures For So3 Sulfur Trioxide Youtube
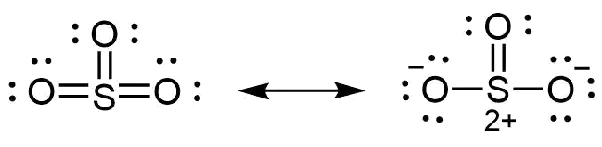
Lewis Dot Atructure Of So3 2 Chemistry Chemical Bonding And Molecular Structure 13065893 Meritnation Com
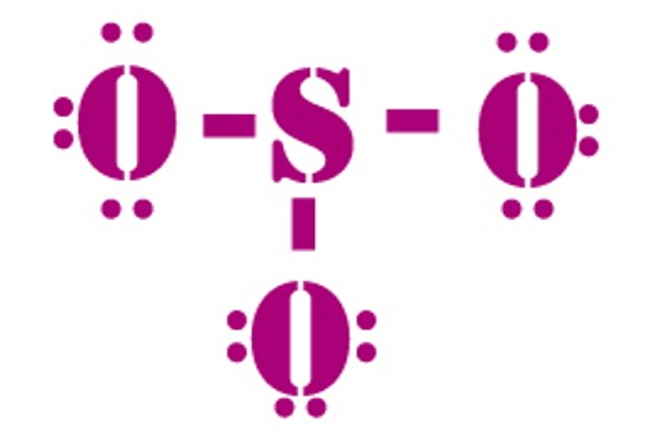
So3 Molecular Geometry Lewis Structure And Polarity Explained

Lewis Structure Of So3 Sulfur Trioxide Youtube

So3 Molecular Geometry Lewis Structure And Polarity Explained
So3 Molecular Geometry Lewis Structure And Polarity Explained

Lewis Dot Of Sulfur Trioxide So3

What Are The Resonance Structures For So3 Quora

So3 Molecular Geometry Lewis Structure And Polarity Explained

How To Determine The Lewis Dot Structure Of So3 Quora

Why So3 Does Not Follow Octet Rule S Could Simply Form A Double Bond With Any One Of The Oxygen Atom Quora