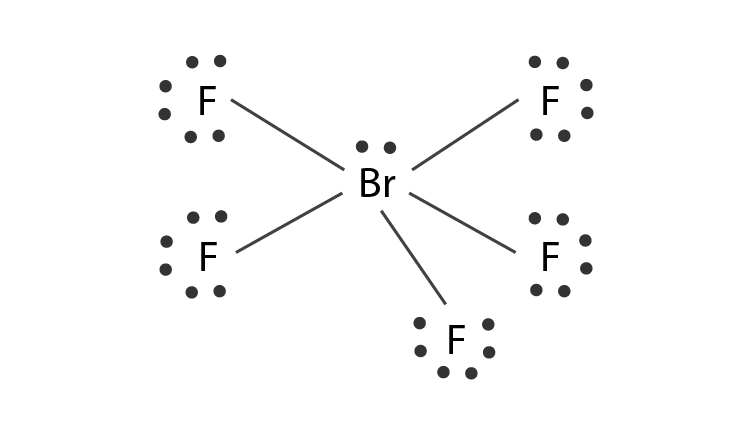
Lewis Dot Structure Of Brf5
It determines the number of outermost valence electrons as well as the electrons engaged in the CH3I molecules bond formation. Next draw the 3-dimensional structure for BrF5 using VSEPR rules.
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5
The electron dot structure of the CH3I molecule is also known as the CH3I Lewis structure.

Lewis dot structure of brf5. BrF5 or bromine pentafluoride has a square pyramidal structure as in the first figure. Total outermost valence shell electrons available for SBr2 Lewis structure dot structure 627 20 valence electrons in SBr2. In the BrF5 lewis structure the total number of electrons is 42 that is found in the periodic table.
The difference between both the values is 102 which is greater. Once we know the number of valence electrons in the BrF5 so after that its easy to distribute them throughout the nuclear atom to achieve the goal of an outer shell of every atom. Once we know how many valence electrons there are in BrF5 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
Want to see the step-by-step answer. For the BrF5 Lewis structure the total number of valence electrons found on the periodic table is 42. Each one of these 3 electrons has the potential to form one single bond with another compound.
The molecular formula for nitromethane is CH3NO2 a. Calculate the total valence electrons in BF 3 molecule. In the Lewis structure for BrF 5 there are a total of 42 valence electrons.
First week only 499. There are a total of 22 valence electrons in the Lewis structure for XeF2. Indicate the hybridization of the.
Start your trial now. What is BrF5 Lewis Dot Structure. You are asking for the Lewis Structure of a compound that cannot exist.
BF5 is Boron Pentafluoride and remember that Boron Pentafluoride has 3 electrons on its outermost shell P-shell. Chemistry QA Library what is BrF5 Lewis Dot Structure. Choose the atom with the least electronegative value atom and insert it in the center of the molecular geometry of SBr2.
Is BrF5 a dipole moment. 8 rows The total valence electron available for drawing the BrF5 lewis structure is 42. According to Tutor Homework the polarity is best found by first drawing the Lewis dot structure for BrF5.
A video explanation of how to draw the Lewis Dot Structure for Bromine Pentafluoride along with information about the compound including Formal Charges Pol. Lewis dot structure of BrF 5. 3 however is possible.
In the BrF 5 Lewis structure Bromine Br is the least electronegative atom and goes in the center of the Lewis structure. What is BrF5 Lewis Dot Structure. Alternatively a dot method can be used to draw the lewis structure of BF 3.
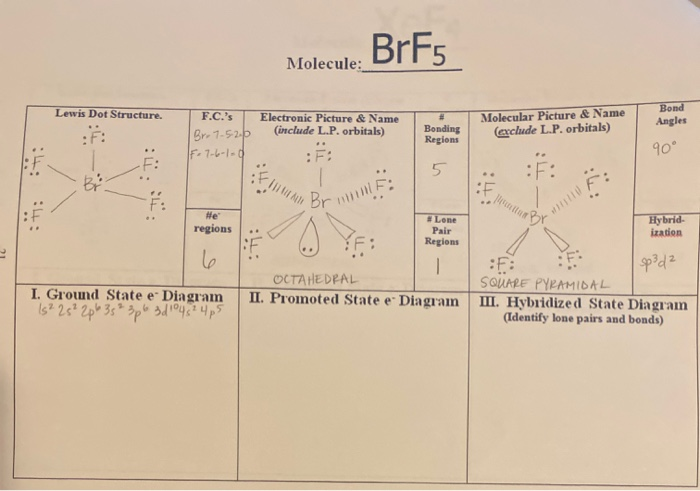
For the BrF 5 Lewis structure youll need to put more than eight valence electrons on the Bromine atom. O 0 l o l b. From the Lewis dot structure of BrF5 it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 296 and 398.
Calculation of total valence electron of SBr2 molecule. Is XeF2 a Lewis structure. But on the other hand BrF5 does have a dipole moment due to the asymmetric structure as shown earlier in the figures.
The problem here is that it cannot be found to 5 Fluorine atoms. Bromine has 35 electrons in atomic structure he can share his 5 electron with fluorine n makes brf5 fluorine has 7 electrone in his outer orbital so he can share 1 eleceone with bromine n complete his orbital by taking 1 electron from bromine but bromine is more powerful than fluorine so he can not gain his electron. Drawing the Lewis Structure for BrF5.
This is a two-dimensional structural representation and gives us a brief yet clear idea about the internal whereabouts of a molecule. Lewis Structure is the diagrammatic form given to the skeleton of any molecular composition or ion formed with the help of the constituent elements the valence electron concept and the bond formation. Valince bonding e a8 non-bonding e.
Draw a valid Lewis structure for this molecule. The molecule has a central bromine atom that is surrounded by five fluorides and a lone pair of electrons. The electron geometry is octahedral and the hybridization is sp3d2.
The outermost valence electrons of the CH3I molecule must be understood while considering the Lewis structure of the molecule. Label one of the oxygens on your structure a and the other b. Note that in the Lewis structure for BrF5 Bromine B is in Period Four on the periodic table.
Use information from step 4 and 5 to draw the lewis structure.

Bromine Pentafluoride Brf5 Is Sometimes Clutch Prep
Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube

Brf5 Lewis And 3 D Structure Dr Sundin Uw Platteville

Make A Sketch Of Brf5 Clutch Prep
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube
How To Determine The Lewis Dot Structure For Bf4 Quora

Brf5 Lewis And 3 D Structure Dr Sundin Uw Platteville

Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Lewis Structure Of Brf5 Biochemhelp
Brf5 El Brillill Molecule Lewis Dot Structure Chegg Com

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

The Central Atom In Brf5 Has How Many Bonding Pairs Of Electrons And How Many Non Bonding Pairs Of Electrons Study Com

Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube

Bromine Pentafluoride Brf5 Lewis Dot Structure Youtube

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube