Lewis Structure Pcl3 Octet Rule
This is what every atom is trying to achieve by making bonds with neighboring atoms. So a transfer of electrons occurs.

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube
Of the outer atoms.

Lewis structure pcl3 octet rule. For molecules before we draw their Lewis structures we have to learn octet rule. Where V 7 5 7 6 7 32 V is the number of valence electrons of the POCl3molecule. Atoms gain lose or share electrons with other atoms in order to fill their valence level with eight electrons.
1 choose a center atom. An electron is promoted into the empty 3d orbital Period 2 elements cannot break the Octet Rule. Subtract the number of bonding electrons from the total.
Only 6 electrons We cant rectify this by drawing three resonance structures. The Octet Rule and Its Exceptions The octet rule states that atoms below atomic number 20 tend to combine so that they each have eight electrons in their valence shells which gives them the same electronic configuration as a noble gas. Find the sum of valence electrons of all atoms 2.
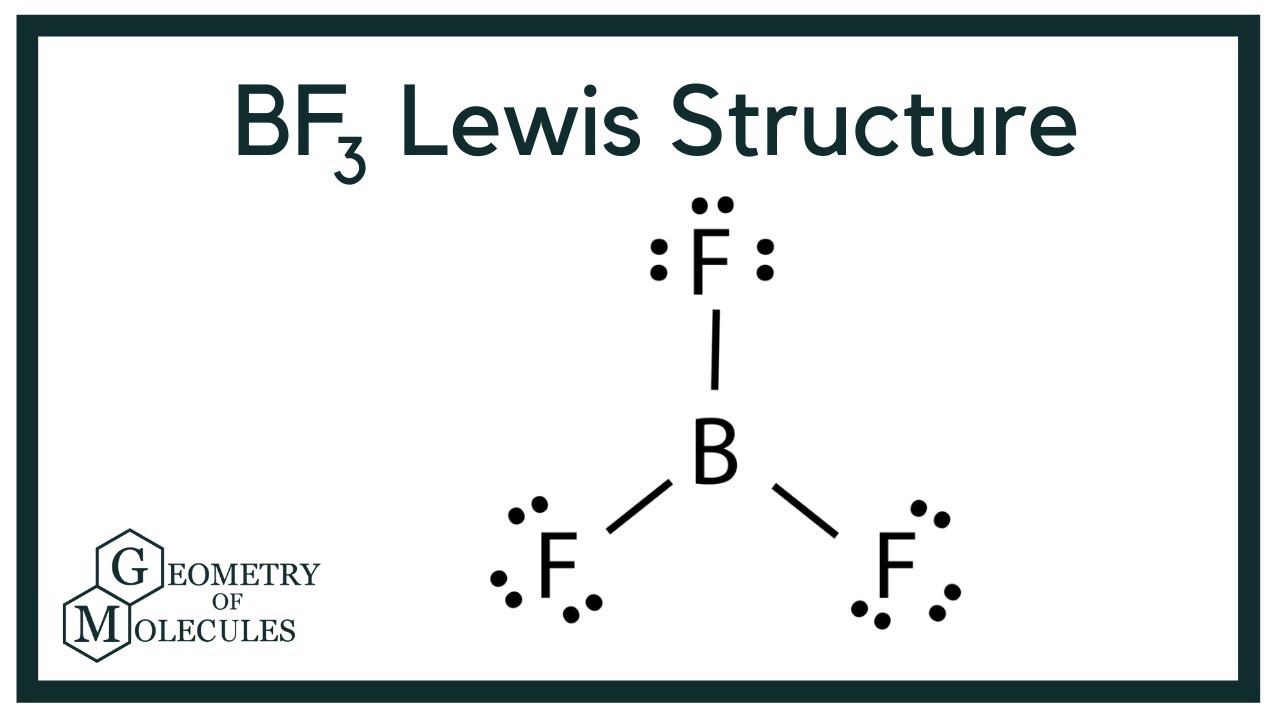
Follow the step-by-step procedure for drawing the Lewis structures. SP only 8 electrons max Period 3 and aboveL you have empty d orbitals to promote electron PCl3 ground state PCl5 excited state Exceptions to the Octet Rule 005026 Boron triflouride BF3. Please watch the following video on how to draw Lewis structures.
What is the octet rule for Lewis structures. When atoms have fewer than eight electrons they tend to react and form more stable compounds. Step 3 4.
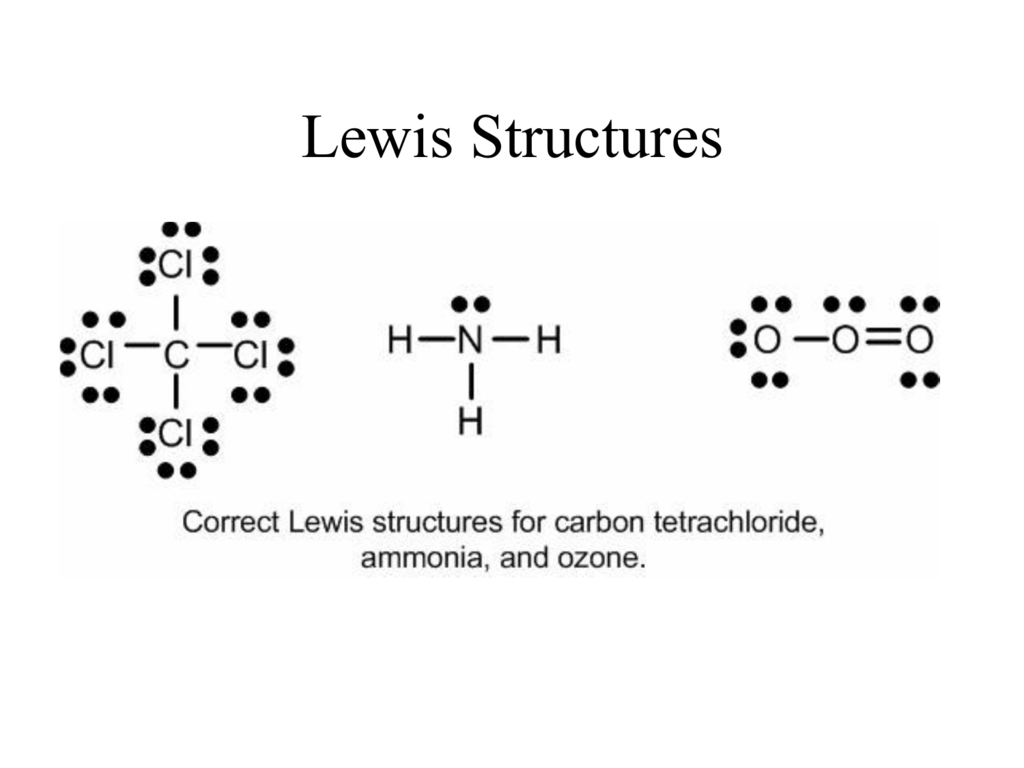
Octet rule states that in forming compounds atoms gain lose or share electrons to give a stable electron configuration characterized by eight valence electrons. Draw Lewis structures step by step. Thus an atom continues to form bonds until an octet of electrons is made.
What is Lewis octet rule. In general you should look at the Lewis dot structures of P and Cl separately and decide by pairing up electrons in P C l 3 to form bonds then checking whether any of the atoms exceed an octet. In this post we discussed the method to construct the CH3I Lewis structure.
CO2 By signing up youll. Octet rule states that in forming compounds atoms gain lose or share electrons to give a stable electron configuration characterized by eight valence electrons. The resonance Lewis electron dot structures of POCl3 are as follows.
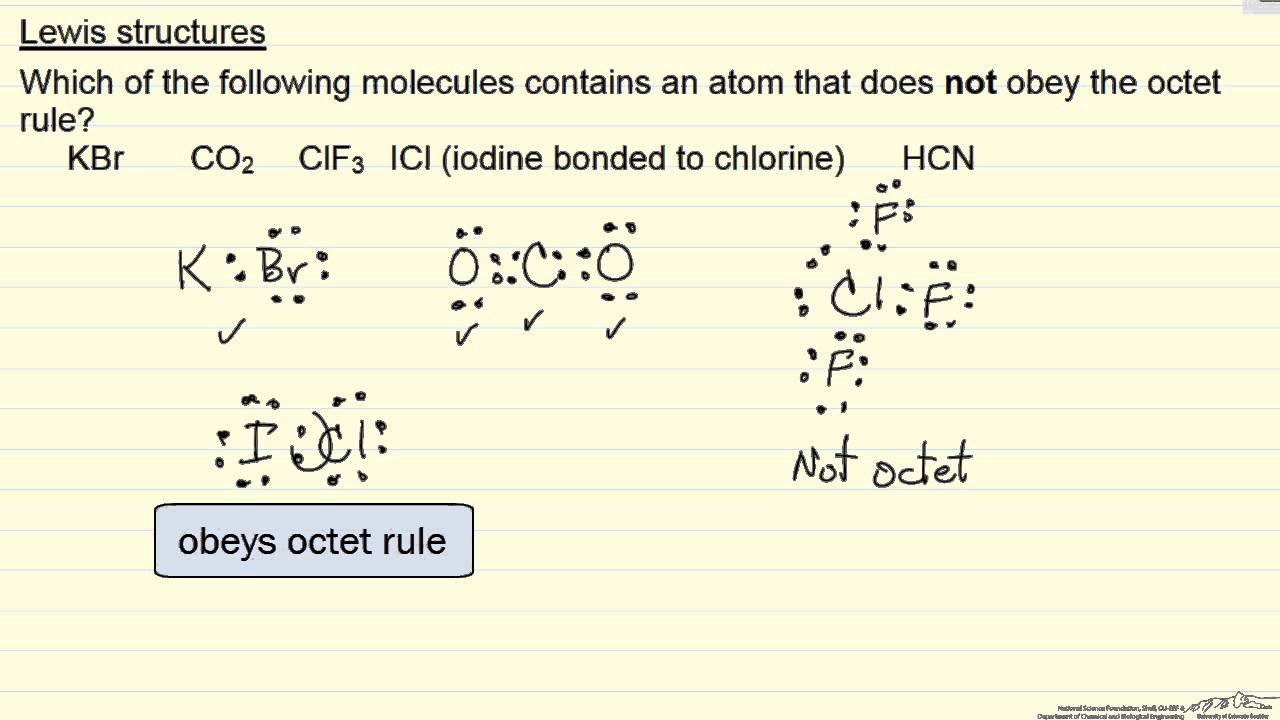
A valid Lewis structure of cannot be drawn without violating the octet rule. The is the least electronegative element that isnt hydrogen. Every atom tries to comply with the octet rule to become stable.
Keep track of the electrons. A valid Lewis structure of _____ cannot be drawn without violating the octet rule. Fill the octet of.
In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. This is known as octet rule by Lewis. Magnesium has a low electronegativity it is a metal after all and fluorine has a high electronegativity it is a non-metal halogen and has the highest electronegativity of all atoms on the table.
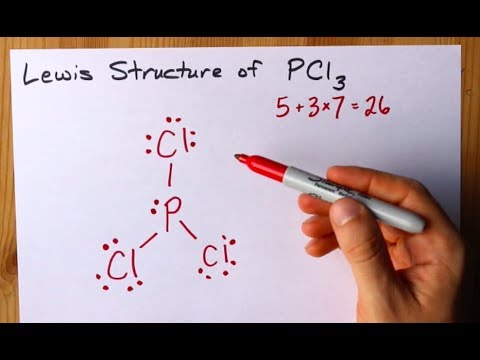
There are a total of 40 valence electrons in SbCl 5 five from Sb atom and 7 from each of five chlorine atoms. Writing Lewis Structures PCl 3 1. Connect the outer atoms to it by single bonds.
If you have done it correctly you will note that phosphorus forms three electron-pair bonds to reach an octet while chlorine forms just one. Electron dot structures of POCl3. First the valence electrons are placed around the carbon atom.
Lewis Structure of Magnesium Fluoride MgF2 Magnesium fluoride MgF2 is not a molecularcovalent compound. Beginning with the n3 principle quantum number the d orbitals become available l 2. This rule is applied to the main-group elements of.
You need 8 electrons in the outermost shell to become stable in nature. Second place the valence electron on the iodine and hydrogen atoms. We subtract 10 electrons to account for the five bonds in the skeleton leaving 30 electrons to distribute.
Therefore P 6n 2 V 6 5 2 32 0 So there is no double bond. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. AClF3 bPCl3 cSO3 dCCL4 eCO2.
Distribute the remaining electrons as lone pairs on the terminal atoms except hydrogen completing an octet around each atom. And that is how different compounds are formed with different and varied chemical and physical properties. Expanded valence shells are observed only for elements in period 3 ie.
The skeletal structure is. This rule is applied to the main-group elements of the second period. Exceptions to the Octet Rule 004546 PCl3 originates from ground state phosphorous PCl5 from the excited state.
This chemistry video tutorial discusses the exceptions to the octet rule while providing the lewis dot diagrams of the molecular compounds involved. The octet rule is based upon available n s and n p orbitals for valence electrons 2 electrons in the s orbitals and 6 in the p orbitals. Connect each atom to the central atom with a single bond one electron pair.

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
Lewis Structures Octet Rule Example Youtube

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science

How Is The Electron Dot Structure Of Pcl3 Determined Quora
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity

6 2 Lewis Structures Introductory Chemistry

Lewis Dot Structure Easy Hard Science
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube

Bf4 Lewis Structure How To Draw The Lewis Structure For Bf4 Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
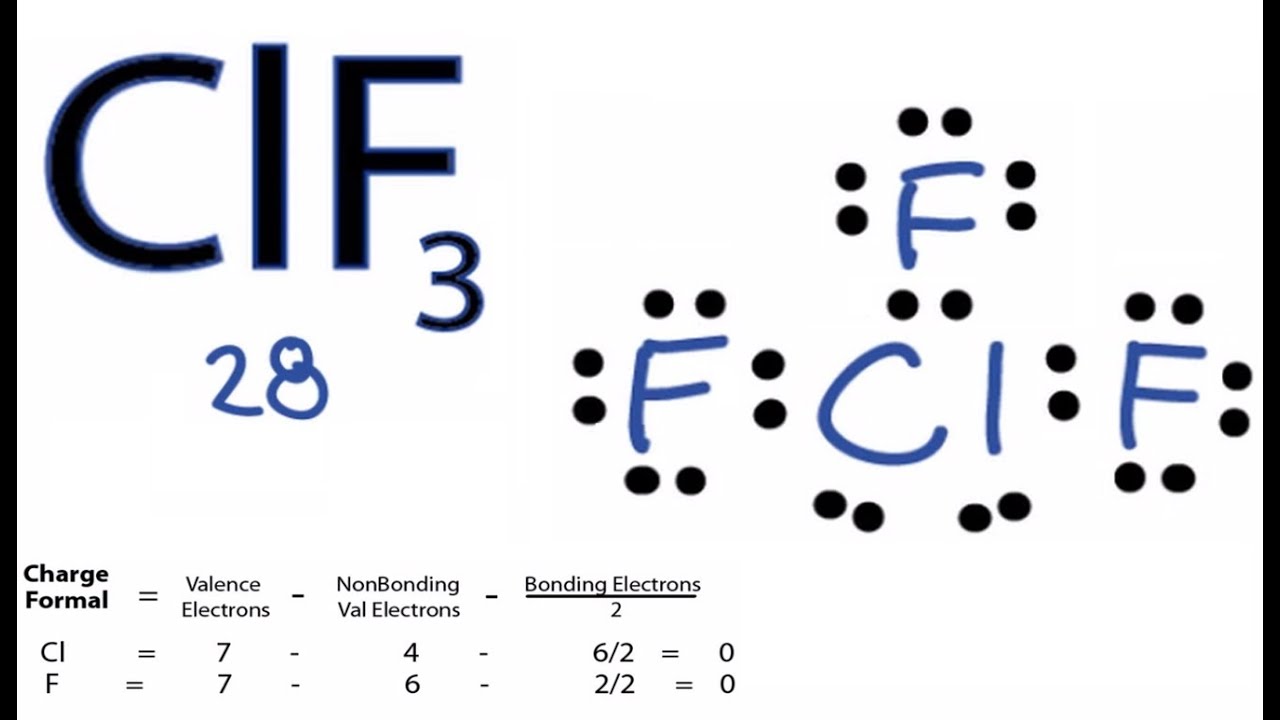
Clf3 Lewis Structure How To Draw The Lewis Structure For Clf3 Youtube

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Lewis Structures For Covalent Molecules Step By Step Youtube

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Chclo Lewis Structure How To Draw The Lewis Structure For Chclo Youtube

Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula