Ncl3 Lewis Structure Hybridization
Hybridization of NF3 is Sp³. What orbitals on N and Cl overlap to form bonds between these elements.

Hybridization For Ncl3 Description Of Hybrid Orbitals For Nitrogen Youtube
PCl3 is a toxic liquid with an unpleasant smell.
Ncl3 lewis structure hybridization. The Lewis structure for NCl3 is. 07 Feb Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms having a chemical formula of PCl3. The total valence electron available for the Nitrogen trifluoride lewis structure is 26.
The hybridization of the AlCl3 molecule is Sp 2 since it has a steric number equal to 3 that will form an Sp 2 hybrid. AlCl3 is a nonpolar molecule because its net dipole moment is zero and charges are uniformly distributed all over the atom. The nature of ICl2- is nonpolar because all dipole that generated along the bond will cancel out because of.
Hello students in this video I explained NO2- NO2 NO3- NO2 lewis structure bond angle NO2lewis dot structure NO2- lewis dot diagram NO2 bond angle. If we consider cesp3 hybrid orbitals of ceNCl3 this tetrahedral arrangement of three σ-bonds and a lone pair disregarding the lone pair its pyramidal is nicely explained. What is the structure of NCl3.
Nitrogen trichloride is a yellow oily liquid with its pungent odor. Our sampling of the monitored swimming pool environments evidenced a mean NCl3. BeBr2 Lewis Structure Geometry Hybridization and Polarity BeBr2 is the molecular formula for Beryllium Dibromide which is a hygroscopic water-soluble compound widely used in x-ray lithography nuclear reactors computer parts catalyst chemical testing and water treatment.
Of valence electron M no. Lewis dot structure of NF3. H ½ VM-CA Here H Hybridization V No.
The geometry of NCl3 is trigonal pyramidal. Hybridization of PCl 3. Along with this concept there is a simple formula for finding hybridization.
The molecular mass of Nitrogen trichloride is calculated as below. What is the hybridization of the nitrogen atom. This is mainly formed as a by-product when chlorine is treated with the ammonia derivative compounds.
NCl3 from the air environment reacts with DPD 3 releasing iodine which reacts with DPD 1 and produces a coloration proportional to the amount of NCl3 from the sampled indoor swimming pool air. The extra unhybridized p orbital is empty but its presence is what keeps the three sp2 orbitals separated by exactly 120 degrees. PCl3 Molecular Electron Geometry Lewis Structure Bond Angles and Hybridization.
Mol mass of NCl3 1 14 mol mass of N 3 3545 mol mass of Cl 120365 gmol. ICl3 Lewis Structure Molecular Geometry Hybridization and Polarity ICl3 named Iodine Trichloride is an Interhalogen compound. To draw the lewis diagram of any molecule we have to follow 5 or 6 simple steps depending on the complexity of the molecule.
Specifically IM allows the entrapment of NCl3 into a water solution containing diethyl-p-phenylenediamine DPD 1 and Potassium Iodide DPD 3. The steric number of iodine central atom in the ICl2- the molecule is 5 thus it forms Sp 3 d hybridization. The N-Cl bonds are formed by the overlap of the ____ hybrid orbitals on nitrogen with the _____ orbitals on Cl.
There is a lone pair of electrons on the nitrogen atom as of the five electrons present in the valence shell of nitrogen atom of NCl3 nitrogen trichloride only three are bonded to the chlorine atom. PCl3 Lewis Structure Hybridization Molecular Geometry and MO Diagram. Molecular Geometry of.
Lewis diagram is a representation of the valence electron within a molecule. If H 2 its Sp hybridization H 3 its Sp2 hybridization H 4 its Sp3 hybridization H 5 its Sp3d hybridization. The molar mass of this compound is 13733 gmol.
What are its electron-pair and molecular geometries. The Lewis Structure of Boron Trichloride BCl3 has three chlorine atoms surrounding a single boron atom. Two lone pairs present on the central atom of the ClO2- Lewis structure.
This liquid can be colorless as well. Less than 109 degrees. Lewis structure of ClO2- contains one single bond and one double bond.
The total valence electron is available for drawing the Aluminium chloride AlCl3 lewis structure is 24. NF3 is polar in nature. Nitrogen and chlorine have the same electronegativity of 30.
Of monovalent atom C charge of the cation A charge of the anion. Thats why the hybridisation is cesp3 which supports every. Interhalogen compounds are molecules which contain at least two different halogen atoms.
This is a trigonal planar arrangement and implies that the boron must be sp2 hybridized. The total valence electron is available ICl2- lewis structure is 22. The molecular geometryVSEPR shape of NF3 is a trigonal pyramid and its electron geometry is tetrahedral.
Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid.

Is Ncl3 Polar Or Nonpolar Explain Study Com

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
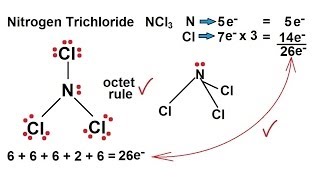
Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Hybridization For Ncl3 Description Of Hybrid Orbitals For Nitrogen Youtube

Page 1 In 2020 Personalized Items Receipt Person

Is Ncl3 Polar Or Nonpolar Quora

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

The Lewis Structure For Ncl3 Is What Are Its Electron Pair Clutch Prep

Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube

Choose A Lewis Structure For Ncl3 Clutch Prep

Determine The Electron Geometry Eg And M Clutch Prep
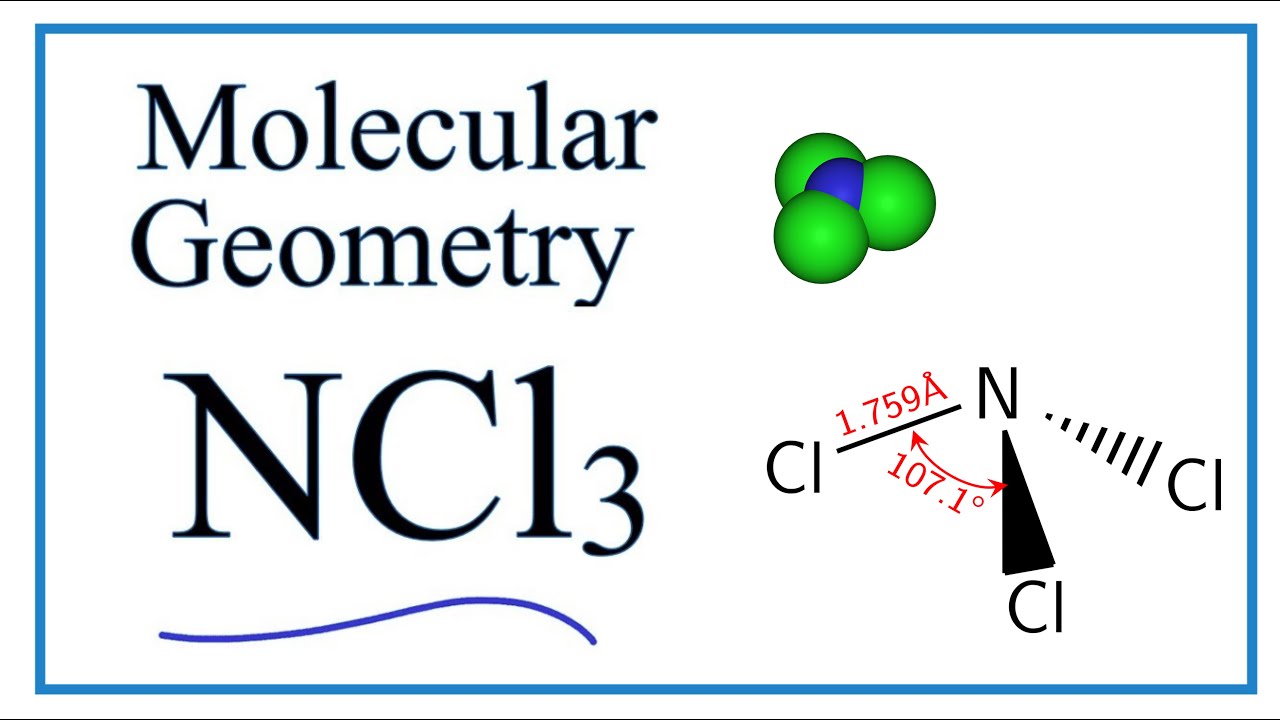
Ncl3 Molecular Geometry Shape And Bond Angles Youtube

Choose A Lewis Structure For Ncl3 Clutch Prep
