Pf3 Lewis Structure Molecular Geometry
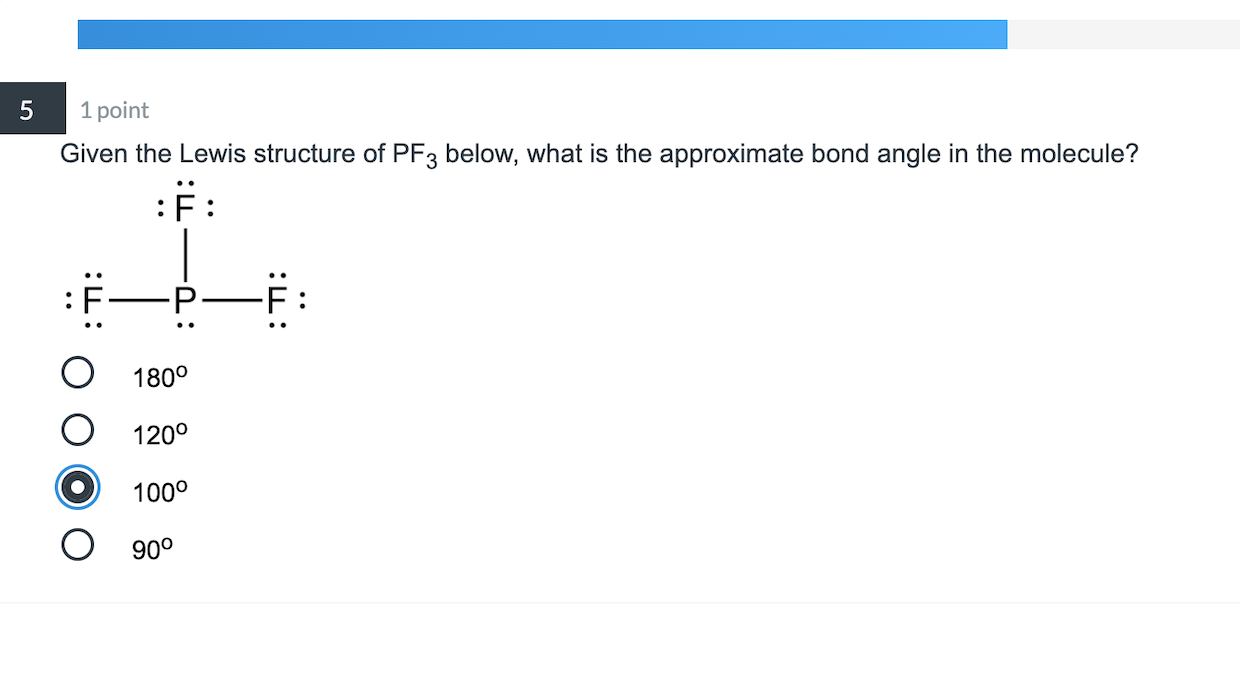
PF3 has 26 valence electrons. Bond angle 1095 Because of the lone pair the bond angle will be less than 1095.
Chemistry Learning Made Easy Pf3 Lewis Structure And Molecular Geometry Youtube
The bond angles for this molecule are 90 and 120.

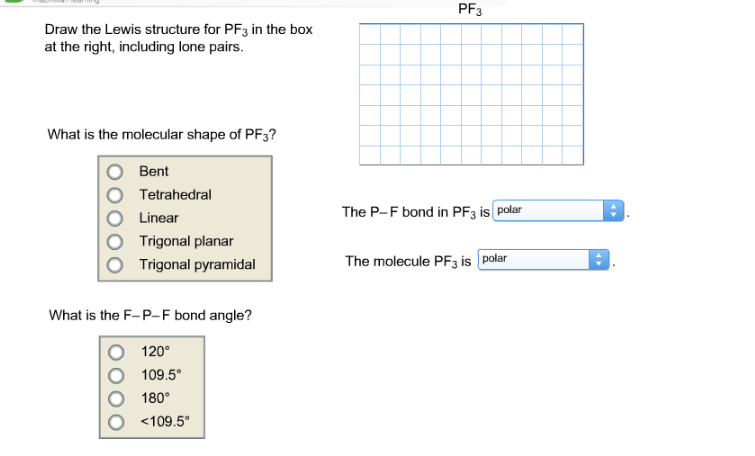
Pf3 lewis structure molecular geometry. Meaning you need four hybrid orbitals to make the molecule. Total outermost valence shell electrons available for SBr2 Lewis structure dot structure 627 20 valence electrons in SBr2. What is the F-P-F bond angel.
Phosphorus Pentafluoride has trigonal bipyramidal molecular geometry with sp3d hybridization. What is the molecular shape of PF 3. It acts as a mild Lewis acid.
Draw the Lewis structure for SF 2 showing all lone pairs. The electron pair geometry ii. 12 Feb 2021 pf3 molecular geometry Uncategorized 0 Comments.
The molecule of PF 3 is polar or nonpolar. Phosphorus will be the central atom because it is less electronegative than Fluorine P will be surrounded by the three F atoms 90 433 ratings. Drawing PF3 Lewis Structure is very easy to by using the following method.
Here in this post we described. Draw a Lewis structure for the molecule. The molecular shape a.
Mol mass of PF3 1 30 mol mass of P 3 189 mol mass of F 8796 gmol. Click to see full answer. We cant really depict the molecules structure in 3D and that is where the molecular geometry helps us.
1095 o 3. Bond angle 1095 Because of the lone pair the bond angle will be less than 1095. However it has some limitations as well.
For each Lewis structure of the following use VSEPR to predict. We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule P F 3 because of its AX3E status. Draw the Lewis structure for PF 3 showing all lone pairs.
Nov 28 2019 As a result they will be pushed apart giving the PF3 molecule a trigonal pyramidal molecular geometry or shape. The outermost valence electrons of the CH3I molecule must be understood while considering the Lewis structure of the molecule. If we talk about the chemical composition of PF3 the molecule consists of one phosphorus atom and three fluorine atoms.
The electron pair geometry ii. We show you how to draw the Lewis structure and determine the moleculargeometry for phosphorus trifluoride PF3. For each Lewis structure of the following use VSEPR to predict.
Hence PF3 has a hybridization of sp3. Pf3 molecular geometry Back to Blog. The valence electrons of Phosphorus are 5 and fluorine has 7 valence electrons in its outermost shell.
Draw a Lewis structure for the molecule. In Sp3 hybridization there are 4 hybrid orbitals 1 s and 3 p orbitals. The Lewis structure of the molecules helps us understand the arrangement of atoms in the molecule.
This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will repel push away from each other in three dimensional space and this gives the molecules their shape. The carbon atom is the middle element in CH3I molecular geometry with four electrons in its outermost valence electron shell whereas the. What molecular geometry would ph3 have.
Correspondingly what Vsepr shape is pf3. There are five single bonds in this molecule as each Fluorine atom forms a bond with the central Phosphorus atom. Solution for Formula Lewis structure Molecule or Electron group Molecular Bond Polarity Ion Type geometry geometry angle PF3 H2O PF3.
PBr3 Molecular Geometry. Lewis structure of Phosphorus Trifluoride PF3 The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. In PF3 phosphorus has one lone pair and three bond pairs which means that total electron pairs is four.
Calculation of total valence electron of SBr2 molecule Choose the atom with the least electronegative value atom and insert it in the center of the molecular geometry of SBr2. PF3 has 26 valence electrons. The molecular mass of PF3 is calculated as.
Moreover is pf5 symmetrical. The phosphorus trifluoride chemical formula is PF3. C r -p -F Determine the total number of electron.
The molecular shape a. VESPR stands for valence shell electron pair repulsion. Draw the Lewis structure of PF 3.
H2CO both H atoms are bonded to C c. What is the hybridization of PF3. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons and three fluorine atoms accept one electron each to undergo a bond formation and reach a stable condition.

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
Pf3 Draw The Lewis Structure For Pf3 In The Box At Chegg Com

Pf3 Molecular Geometry Shape And Bond Angles Youtube
5 1 Point Given The Lewis Structure Of Pf3 Below Chegg Com

What Is The Molecular Geometry Of Pf3 Study Com

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have

Chemistry Learning Made Easy Pf3 Lewis Structure And Molecular Geometry Youtube

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Predict Molecular Geometry Of Pf3 Phosphorus Trifluoride Youtube

Answer The Molecular Geometry Of Pf3 Is B Clutch Prep

Pf3 Molecular Geometry Shape And Bond Angles Youtube
Pf3 Molecular Geometry Shape And Bond Angles دیدئو Dideo

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
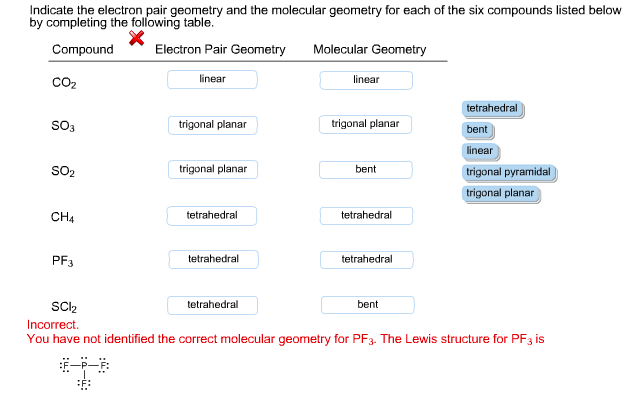
Indicate The Electron Pair Geometry And The Molecular Chegg Com

Pf3 Draw The Lewis Structure For Pf3 In The Box At Chegg Com

Pf3 Molecular Geometry Shape And Bond Angles Youtube

What Is The Electron Geometry Of Pf3 A Bent Angular B T Clutch Prep

A What Is The Molecular Geometry Of Pf3 Clutch Prep