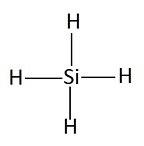
Sih4 Lewis Diagram
Silicon is in Group 14. Start your trial now.

Lewis Symbols And Structures General Chemistry Lecture Lab
Calculate the total number of valence electrons present.

Sih4 lewis diagram. Silane is an inorganic compound with chemical formula SiH4. What is the Lewis dot structure for silicon. A stepbystep explanation of how to draw the sih4 lewis structure silicon tetrahydride.
Draw the Lewis structure of SiH4. Determine the central atom in this molecule. What is the Lewis structure of SiH4.
SiH4 Lewis Structure Lewis Structure is a two-dimensional diagrammatic approach towards finding the nature of chemical bonding present inside any given molecule. This obeys the valence shell electron pair repulsion theory in which. 3 1 h2o 104 5 o 2 2 draw lewis structure and find number of pairs of electron determine electron pair geometry determine molecular geometry the total number of nonbonding electron pairs present in the lewis structure of sih4 is a zero b one c 1 13.
What is the Lewis dot structure for SiH4. Include lone pairs as needed. 8 electrons of the total valence.
You must refer to SiH4 Lewis Structure Geometry Hybridization. Question Is SiH4 polar or nonpolar. The net dipole moment of silane is zero and it is non-polar.
Free unlimited access for 30 days limited time only. Chemistry QA Library Draw the Lewis structure of SiH4. It is a colourless flammable gas with a sharp repulsive smell somewhat similar to that of acet view the full answer Previous question Next question.
Put the Si in the center Hydrogens always go on the outside. To do so we first need to do the following steps. Include lone pairs as needed.
Include lone pairs as needed. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Draw the Lewis structure of SiH4.
Draw the Lewis structure of SiH 4. It provides molecular geometry bond angle and hybridi. Both use all 20 valence el.
Start your trial now. First week only 499. Lewis Diagram for SiH4.
Lewis structure presents the structural formation of the atoms in space which form the least strain. Chemistry learning made easyThis tutorial will help you deal with the lewis structure and moleculargeometry for silane SiH4. Hydrogen group 1 1 valence electron but we have four of them.
Lets do the Lewis structure for SiH4. Since hydrogen has a higher electronegativity than silicon the polarity of the bond between silicon and hydrogen is the inverse of that of. Hydrogen only needs two electrons to have a full outer shell so each of the Hydrogens is fine.
Draw a Lewis structure for silane SiH4 and predict its molecular geometry. So 4 plus 4. First week only 499.
Answer SiH4 silane is Nonpolar What is polar and non-polar. Draw the Lewis structure of SiH4. This video shows you how to draw the lewis dot diagram structure for sih4 silicon tetrahydride.
In the periodic table silicon group 4 4 valence electrons hydrogen group 1 1 valence electron but we have four. It is easily ignited in air reacts with oxidizing agents is very toxic by inhalation and is a strong irritant to skin eyes and mucous membranes. Were being asked to draw a Lewis structure for SiH 4.
On the periodic table Silicon group 4 4 valence electrons. Silane is a colorless flammable and poisonous gas with a strong repulsive odor. It has a full outer shell.
Chemistry QA Library Draw the Lewis structure of SiH4. Include lone pairs as needed. 8 total valence electrons.
Put Yes in the middle hydrogen always comes out. Silane can be considered as Methanes silicon analog. Molecules sih4 lewis structure how to draw the dot structure for hw 6 flashcards quizlet lone pair wikipedia dressen lewis structures authorstream lewis structures octet rule a simple method to write solved what is the number of bonding electron pairs on th.
Draw the Lewis structure for SiH4. Include lone pairs as needed. Here we use dot notations to represent the electrons and hence this is also known as the electron-dot structure.
So that is the SiH4 Lewis structure. Get the detailed answer. Silane is lighter than air.
Include lone pairs as needed. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. So 4 plus 4.
Lets do the Lewis structure for SiH4. And the Si needs eight which it has.

How To Draw Lewis Structure For Sih4 Drawing Easy
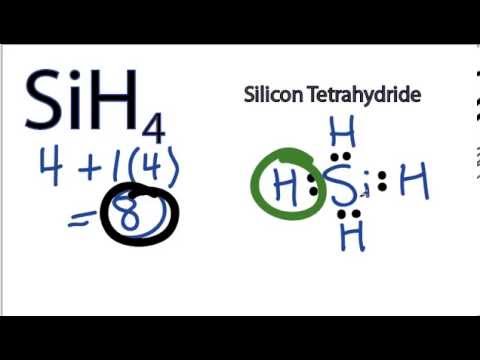
Sih4 Lewis Structure How To Draw The Lewis Structure For Sih4 Silicon Tetrahydride Youtube

Draw The Lewis Structure For Sih4 Clutch Prep
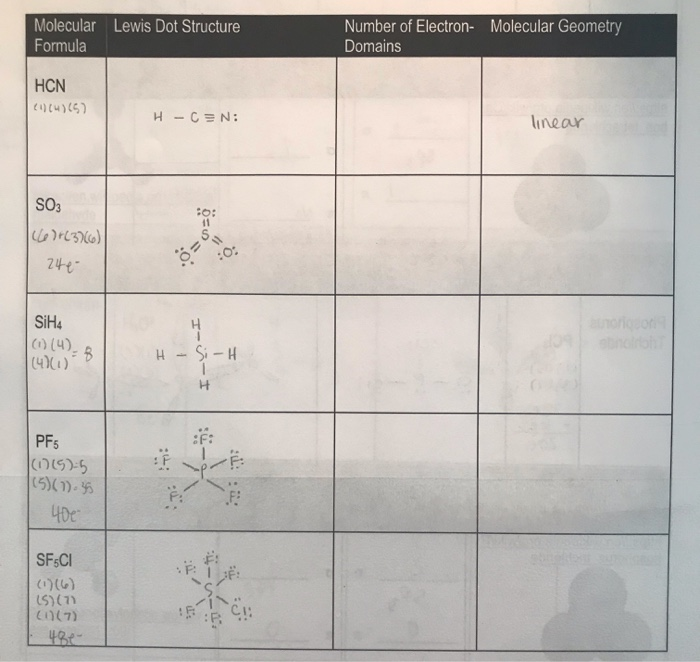
Molecular Formula Lewis Dot Structure Number Of Chegg Com
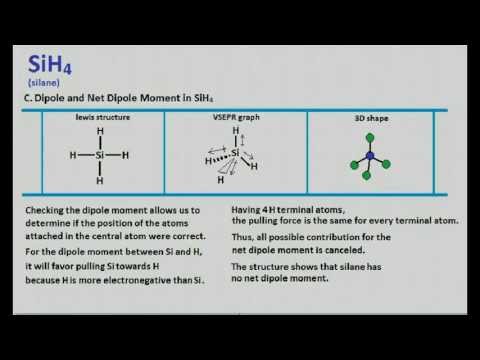
Sih4 Lewis Structure Molecular Geometry Youtube

What Is Sih4 S Shape Study Com

Sih4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Draw A Lewis Structure For 1 Sih4 2 Brf3 3 H2co You Must Use All 5 Steps In The Process For Drawing Lewis Structure Study Com

Sih4 Lewis Structure Molecular Geometry Youtube
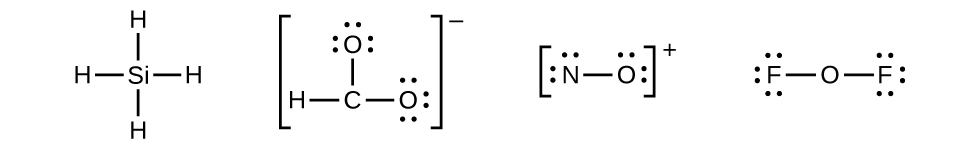
4 2 Lewis Structures Chemistry Libretexts

Quiz Worksheet Lewis Structures Study Com

Is Sih4 Polar Or Non Polar Silicon Tetrahydride Youtube

Sih4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Sih4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Answered Draw The Lewis Structures And Write The Bartleby

Write A Lewis Structure For Each Molecule Sih4 Draw The Mo Clutch Prep
Answered Draw Lewis Structures For The Bartleby

Draw A Lewis Structure For Silane Sih4 A Clutch Prep
