What Is The Molecular Geometry Of Pcl5
Molecular Weight Molar Mass. Geometry of PCl5 is trigonal bipyramidal.
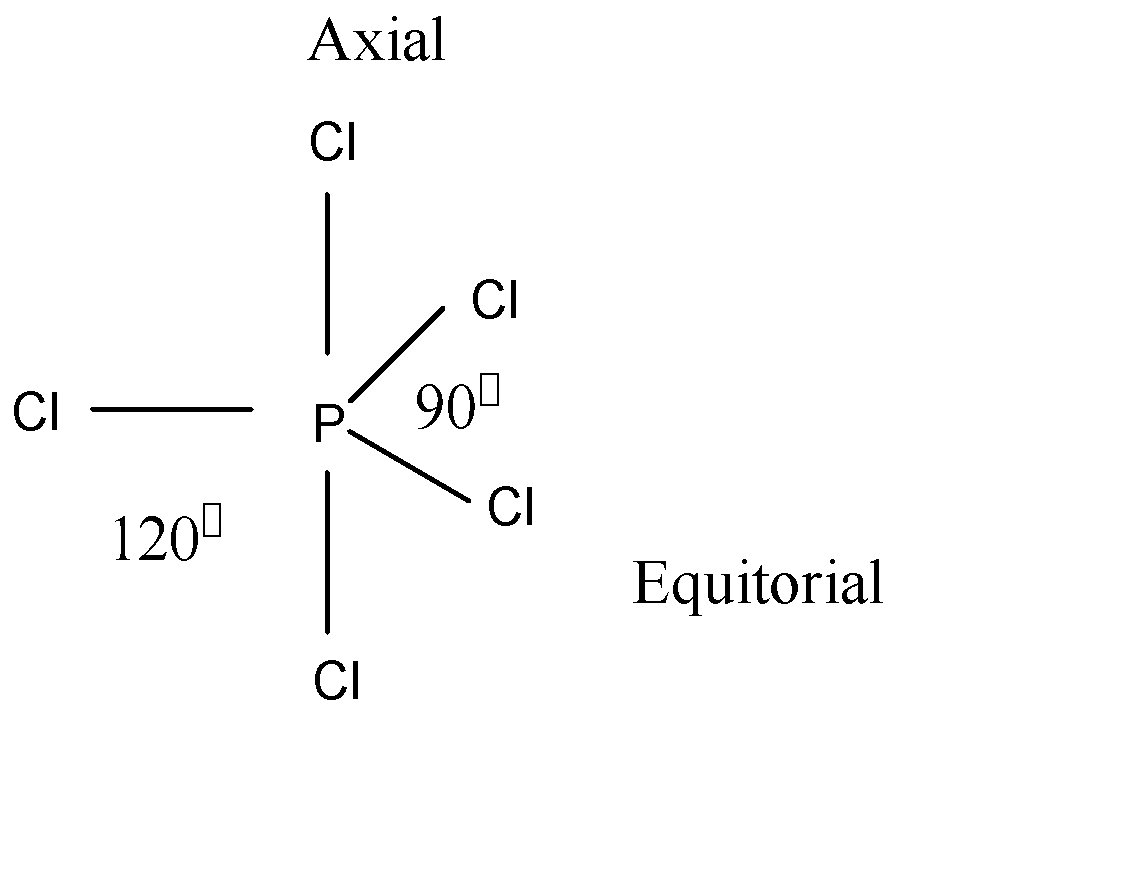
Cl P Cl Bond Angles In Pcl5 Molecule Is A 120 Circ Class 11 Chemistry Cbse
The molecular geometry of XeO3 is trigonal pyramidal with asymmetric charge distribution on a central atom.

What is the molecular geometry of pcl5. What shape is PCl5. VSEPR is short for valence shell electron pair. Furthermore How do you find the molecular geometry Steps Used to Find the Shape of the Molecule Draw the Lewis Structure.
See full answer below. As there are 5 bonds of Cl. PCL5 has five electron domains without lone pairs of electrons on its central atom.
SO2 has four electron domains leading to a tetrahedral electron domain geometry according to valence shell electron pair repulsion theory. What are the bond angles for square pyramidal molecular geometry. Phosphorus has 5 valence electrons.
What is the shape molecular geometry of PCl3. What is shape of XeF4 xeo3. What will be the hybridisation of PCl5.
The geometry of XeF4 is a square planar with symmetric electron reigon distribution. Furthermore What is the molecular shape of NCl3 Hence the Geometry of the molecule of NCl3 is Trigonal pyramidal. The Lewis structure of PCl 3 is.
The steric number of the central P atom in P Cl5 P C l 5 is 5 because the central P atom is forming five P-Cl bonds and doe not have any lone pair of electrons. 3 in same plane and one in upper and another in down palne. 1 electron each is shared by chlorine atom hence PCl5 molecule has sp3d hybridisation and hence trigonal bipyramidal shape.
6 Zeilen PCl 5. C 0 votes. The molecular geometry of PCl 3 is trigonal pyramidal.
What is the shape molecular geometry of PCl3 The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom. Asked Jun 26 2017 in Chemistry by Kabecilla. Bond angle is 900 and 1200.
Geometry of PCl5 molecule is trigonal bipyramidal. So the hybridization of the central. When we consider both lone pairs and bond pairs we are referring to the Structure of the molecule.
What is the shape of PCl5 according to Vsepr theory. Therefore XeF4 molecular geometry is square planar. However the lone pairs on the central atom in the molecule leads to a bent molecular geometry.
What is the shape molecular geometry of PCl3 The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom. So it makes triangle when 3 are in same plane. Answered Jun 26 2017 by SaltyBones.
Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs.

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
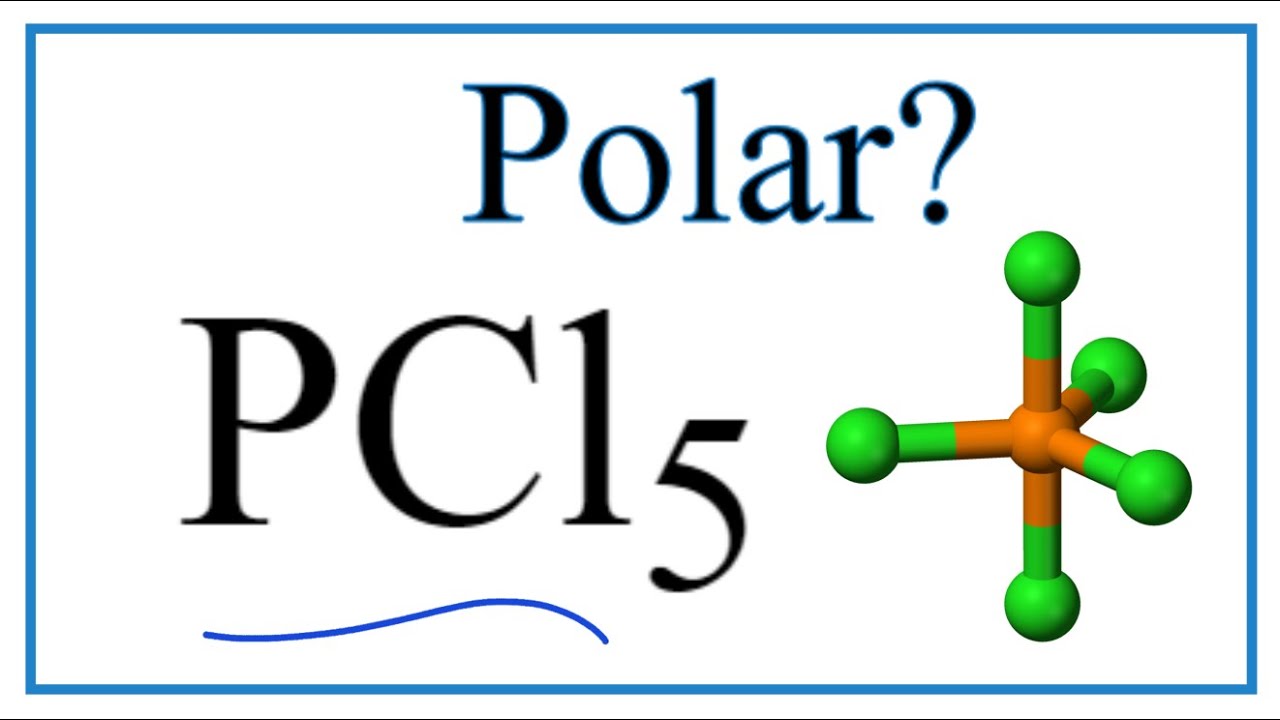
Is Pcl5 Polar Or Nonpolar Phosphorous Pentachloride Youtube
What Is The Geometry Of Pcl5 Quora
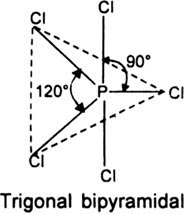
On The Basis Of Vsepr Theory Predict The Shapes Of Given Molecules Pcl5 And Sf6 From Chemistry Chemical Bonding And Molecular Structure Class 11 Haryana Board English Medium
What Is The Molecular Geometry Of Pcl5

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
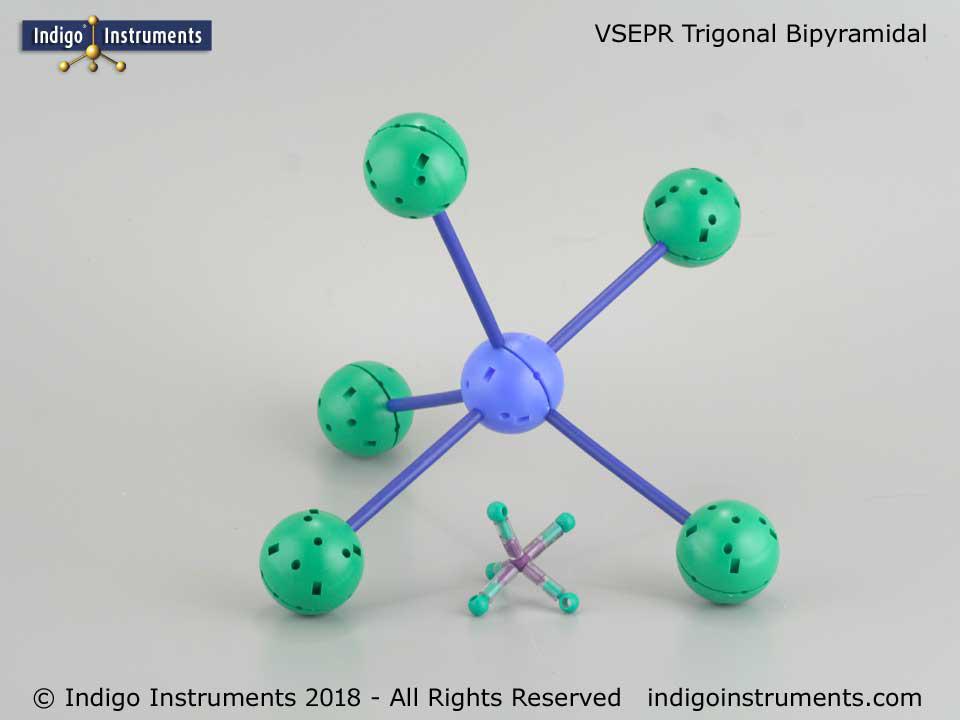
Pcl5 Trigonal Bipyramidal Molecular Geometry Vsepr Theory Model
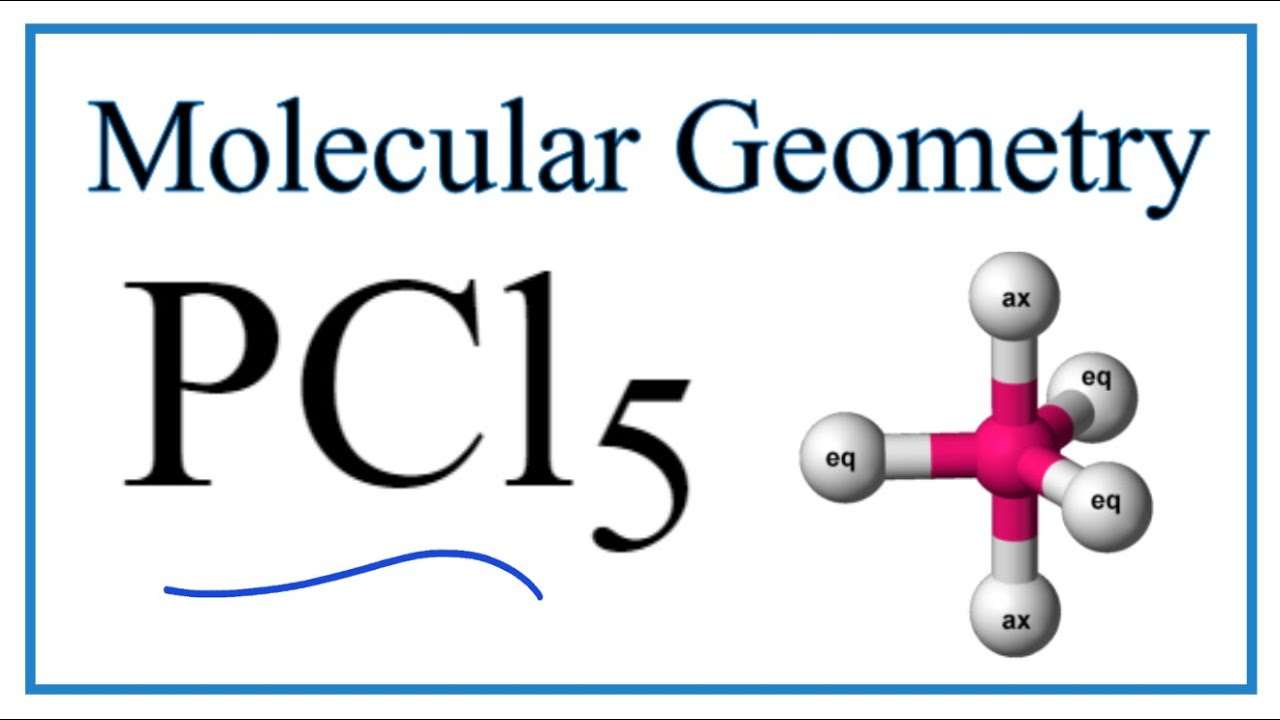
Pcl5 Phosphorous Pentachloride Molecular Geometry Bond Angles Youtube

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

What Is The Molecular Geometry Of Pcl5 Quora

Pcl5 Hybridization Trigonal Bipyramidal With Sp3d Hybridization On Byju S
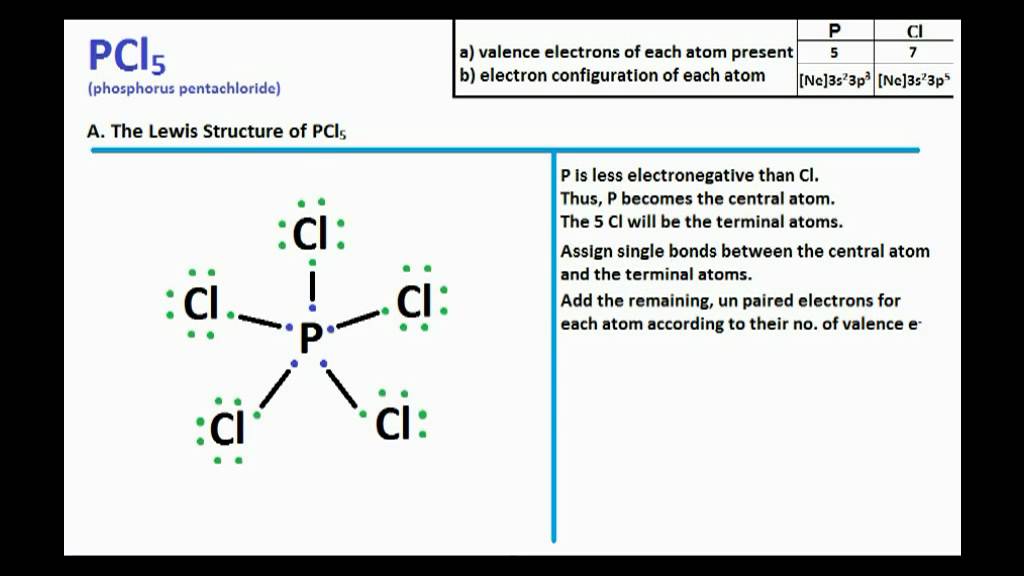
Pcl5 Lewis Structure And Molecular Geometry Youtube

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
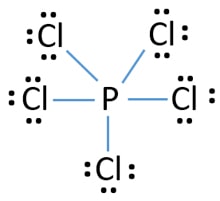
Pcl5 Phosphorus Pentachloride Lewis Structure
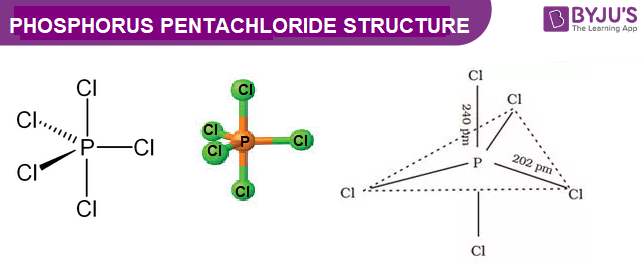
Pcl5 Phosphorus Pentachloride Structure Molecular Mass Properties And Uses

Pcl5 Villanova College Chemistry Blog
Explain The Structure Of Pcl3 And Pcl5 Chemistry Topperlearning Com C9yvmoxx