Xef2o Polar Or Nonpolar
Alright guys let us know. Question Is XeO2F2 polar or nonpolar.

Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples Covalent Bonding Chemical Bond Study Chemistry
Now is XeF2 polar or nonpolar.

Xef2o polar or nonpolar. Your email address will not be published. XeO2F2 is polar and it helps to identify this through drawing out the lewis structure. Nonpolar compounds either have no polar bonds or contain symmetrical polar bonds.
Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. However Xe-F bond is polar because the electronegativity of Xe and F is different but the polarity of both Xe-F bonds gets canceled by each other resulting in a nonpolar XeF2 molecule. This is because XeF4 has an octahedral symmetric geometry.
Is XeF4 Polar or Non-polar. Xef2 polar or nonpolar. As these are a pure covalent bond so they are insoluble in water called hydrophobic and other polar solvents but are soluble in nonpolar solvents like carbon tetrachloride CCl4 carbon dioxide CO2 benzene C6H6 etc.
Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Thus the molecule is polar. Now Is XeF4 polar or nonpolar.
Xenon Tetrafluoride - YouTube. A polar molecule with two or more polar. It is made of one Xenon atom and two Fl.
If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. A polar molecule with two or more polar bonds must. Posted by MakeTheBrainHappy on April 14 2020 Get link.
The polarity induced on four Xe-F. Why is XeF2 a nonpolar molecule. XeF2 is a nonpolar molecule despite two Xe-F bonds are polar.
Learn to determine if XeF4 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and then. The molecule is therefore non-polar. Xef2 polar or nonpolar.
Answer XeO2F2 is Polar What is polar and non-polar. But due to the linear structure ie. List molecules polar and non polar.
The single lone pair gives the compound a seesaw shape which then would be polar. The terms polar and nonpolar usually refer to covalent bonds. So Is XeF2 Polar or Nonpolar.
If the result is between 04 and 17 then generally the bond is polar covalent. Posted by MakeTheBrainHappy on April 14 2020 Get link. How do I know if a bond is polar or nonpolar.
In your example of SF_4 the Lewis Structure would look like this. To determine the polarity of a covalent bond using numerical means find the difference between the electronegativity of the atoms. Symmetrical the dipoles of both Xe-F bonds get canceled by each other resulting in a nonpolar XeF2 molecule.
Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. XeF2 is a nonpolar molecule due to the linear arrangement of the fluorine atoms around the central xenon atom.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. If it is not completely symmetrical AND it has polar bonds it will be polar. Xef2 polar or nonpolar.
You can see that there is a lone. In doing so you can see that Xe has 5 electron densities with 4 bonded pairs and one lone pair. Hey Guys In this video we are going to determine the polarity of Xenon Difluoride having a chemical formula of XeF2.
February 13 2021 in Uncategorized in Uncategorized. Well moreover the polar solvents possess molecules with polar bonds and nonpolar solvents possess molecules with similar electronegativity valuesThe easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and if necessary check its molecular geometry. XeF4 is a nonpolar molecule despite four individual Xe-F bonds are polar.
The XeF2 has a linear molecular geometry and Xe-F bonds are symmetrical to each other as a result the net dipole moment becomes zero. Ill tell you the polar or nonpolar list below. So overall uh this molecule is going to be non polar with polar bonds of course but its going to be numb pull or due to its geometry the way that the bonds and the lone pairs arrange themselves this is going to be a non polar molecule.
Is XeF2 polar or nonpolar. XeF2 is nonpolar in nature because of its linear-shaped geometry having fluorine atoms symmetrically on both sides of the xenon atom. XeF2 is Nonpolar Ill tell you the polar or nonpolar list below.
Learn to determine if OF2 Oxygen difluoride is polar or non-polar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis S. Answer XeF2 Xenon difluoride is Nonpolar What is polar and non-polar.

Difference Between Polar Covalent Bond And Non Polar Covalent Bond Covalent Bonding 11th Chemistry Bond
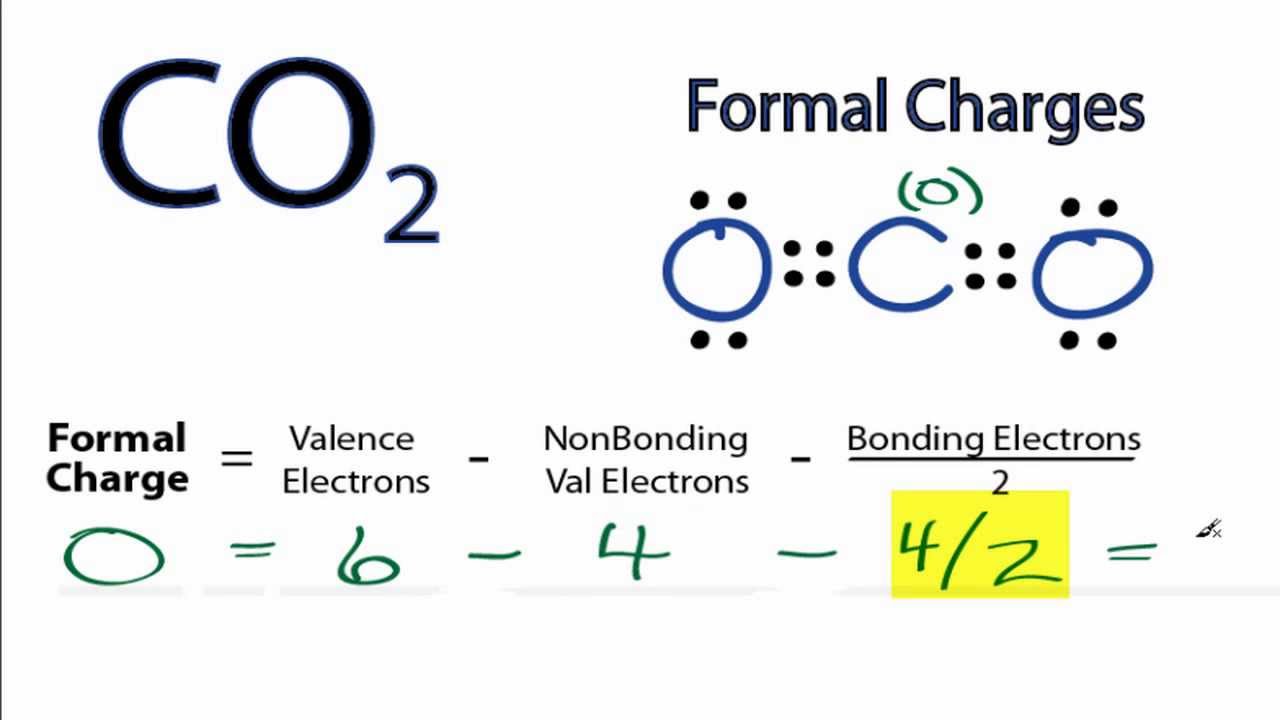
Calculating Co2 Formal Charges Calculating Formal Charges For Co2 Youtube

Xef2o Lewis Structure Xenon Oxytetrafluoride Youtube
A Quantitative Definition Of Hypervalency Chemical Science Rsc Publishing Doi 10 1039 C5sc02076j

Chf3 Lewis Structure How To Draw The Lewis Structure For Chf3 Youtube
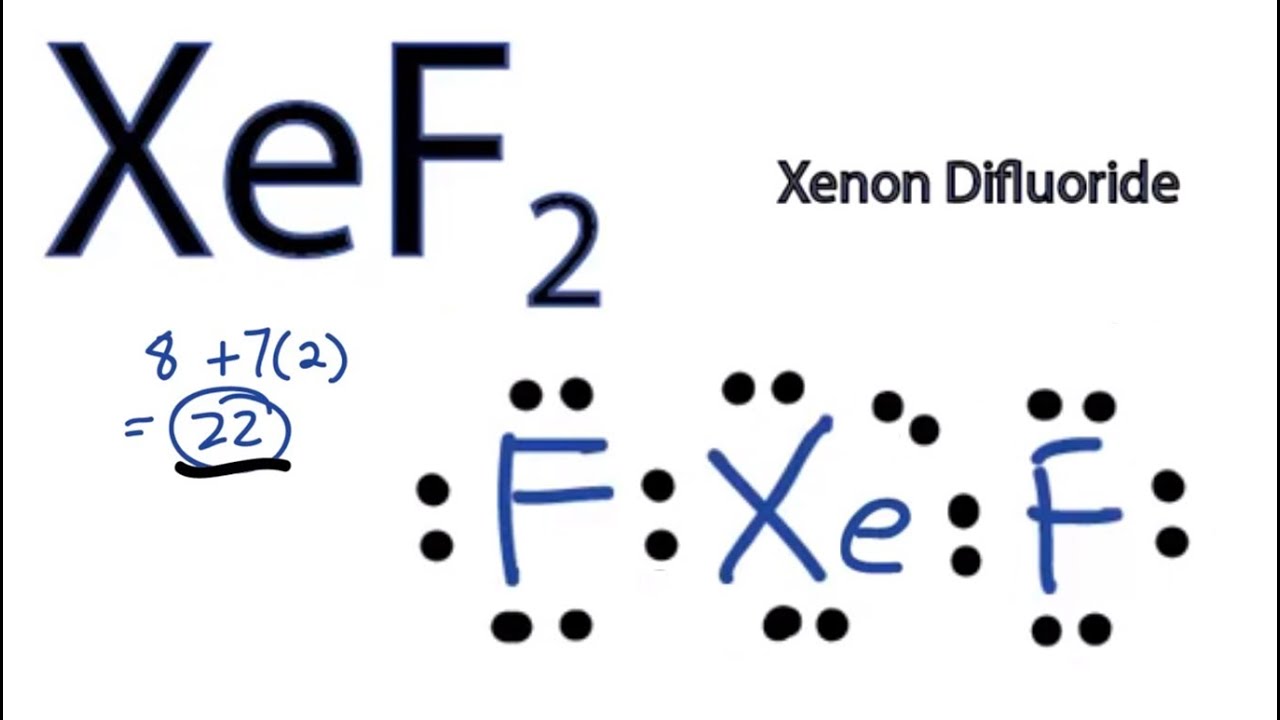
Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 Youtube

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Xef2o Lewis Structure Xenon Oxytetrafluoride Youtube
How To Draw An Xef2o Lewis Structure Quora
Why Is The Lewis Structure Of So2 Not Similar To 03 Quora

Ash3 Lewis Structure How To Draw The Lewis Dot Structure For Arsenic Trihydride Youtube

Xef2o Lewis Structure Xenon Oxytetrafluoride Youtube

How To Draw An Xef2o Lewis Structure Quora

Is Chcl3 Polar Or Nonpolar Polar Molecules Energy Flow

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 Youtube

Xef2o Lewis Structure How To Draw The Lewis Structure For Xef2o Youtube

How To Draw An Xef2o Lewis Structure Quora

How To Draw The Lewis Dot Structure For Pscl3 Youtube