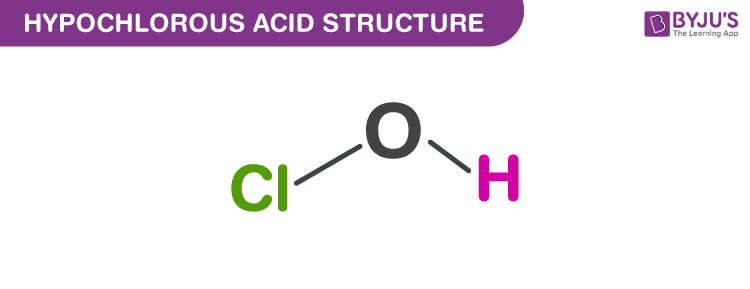
Draw The Lewis Structure Of Hocl
Hydrogen shares one electron with. The properly way to determine the Lewis structure based on this example is.

What Is The Lewis Structure Of Hypochlorous Acid Youtube
Drawing the Lewis Structure for HOCl This means that even though Oxygen atom is more electronegative than Chlorine it will still be placed at the center of the HOCl Lewis structure.
Draw the lewis structure of hocl. Steps of drawing lewis structure of HOCl Find total number of electrons of the valance shells of hydrogen oxygen and chlorine atoms Determine total electrons pairs as lone pairs and bonds Find center atom and basic sketch Mark lone pairs on atoms Mark charges on atoms if. The molecular geometry is described only by the positions of the nuclei not by. Draw a Lewis structure for each of the followinga.
7 6 1 14 Total electrons needed for octetsdoublets. Lewis theory Gilbert Newton Lewis 1875-1946 focuses on the valence electrons since the outermost electrons are the ones that are highest in energy and farthest from the nucleus and are therefore the ones that are most exposed to other atoms when bonds form. First we need to count the valence electrons of hydrogen H chlorine Cl and oxygen O.
C2Br2Draw a Lewis structure including the resonance forms for each of the following molecules or ionsa. C2Br2 Draw a Lewis structure including the resonance forms for each of the following molecules or ions. Write Lewis structures for Br2 CH4 and HOCl.
Hocl lewis structure. In this section we will learn how to draw the Lewis structure of hypochlorous acid. You have a total of 8 valence electrons available to fill the octets of.
This is the HOCl Lewis structure. Well put two between atoms to form chemical bonds. This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule.
For HOCl we have 1 valence electron plus 6 for the Oxygen and Chlorine has 7 for a total of 14 valence electrons. A weak unstable acid it is the active form of chlorine in water. 8cdot22cdot1 18 Total sharedbonding electrons.
Thus the Lewis structure of HOCl is. I see that HOCl has V-sharp and its structure is H O Cl. We draw Lewis Structures to predict-the shape of a molecule-the reactivity of a molecule and how it might interact with other molecules-the physical properties of a molecule such as boiling point surface tension etc.
We have 14 valence electrons for the HOCl Lewis structure. A step-by-step explanation of how to draw the HCl Lewis Dot Structure Hydrochloric acidFor the HCl structure use the periodic table to find the total numb. A step-by-step explanation of how to draw the correct Lewis Dot Structure for Hydrogen chloride HCl gasFor the Hydrogen chloride structure use the periodi.
Hydrogen and Chlorine are both non-metals so they form a COVALENT bond and SHARE electrons to complete their outer shells. For the HOCl Lewis structure there are a total of 14 valence electrons. What is the molecular shape of HOCl.
Hypochlorous acid is a chlorine oxoacid with formula HOCl. DO NOT FORGET TO SUBSCRIBELinkedIn. This is the HOCl Lewis structure.
Is the molecule HOCl. 18-14 4 In other words there are only two single bonds And Chlorine also has an octet. The best way to arrange.
It has a role as a human metabolite an EC 3117 acetylcholinesterase inhibitor and an EC 25118 glutathione transferase inhibitor. Put the H here--always goes on the outside--and then the Oxygen and the Chlorine. The central oxygen atom has 4 electron groups around it which are 2 single bonds and 2 lone pairs.
OCl2 oxygen dichloride Cl O Cl VSEPR geometry. For HOCl we have 1 valence electron plus 6 for the Oxygen and Chlorine has 7 for a total of 14 valence electrons. For oxygen 2 lone pairs are drawn.
Hocl Lewis Structure How To Draw The Lewis Structure For Hocl Youtube. Draw a Lewis structure for each of the followinga. Then around the outside atoms so well have 4 6 8 10.
Drawing the Lewis Structure for HCl Hydrochloric Acid Another straight forward Lewis structure. Sodium hypochlorite in 05 wv solution is called Dakins solution and is used as an antiseptic to clean infected topical wounds. Lewis structure for HOCl.
C2Br2Draw a Lewis structure including the resonance. HOCl is called Hypochlorous Acid.

What Is The Lewis Structure For Hclo Chemistry Stack Exchange

Hclo Definition Lewis Structure Video Lesson Transcript Study Com

No More All Nighters Structural Formula Chemistry Education Nurse Drawing

Remember Ions Are Atoms Or Groups Of Atoms With A Charge Ppt Download
Hypochlorous Acid Wtinternational

What Are Hydrogen Bond Accepter And Hydrogen Bond Donor Hydrogen Bond Science Chemistry Education Science

Which Of The Following Is Industrially Prepared By Passing Ethylene Into Hypochlorous Acid Youtube

Oneclass Draw Two Possible Lewis Structures For Hypochlorous Acid With The Atoms Arranged As Written
Hypochlorous Acid Hocl Is Used In Water Treatment And As A Disinfectant In Swimming Pools A0 150 M Solution Of Hocl Has A Ph Of 4 18 What Is The Ph For Ka Hypochlorious

Hclo Definition Lewis Structure Video Lesson Transcript Study Com
Hypochlorous Acid Wtinternational

Hocl Lewis Structure How To Draw The Lewis Structure For Hocl Youtube
Hypochlorous Acid Structure Properties Uses Of Hocl
Hypochlorous Acid Hclo Pubchem




