The Best Lewis Structure For Co2
O2 Best Lewis Structure Nbr3 Lewis Structure Awesome Lewis Structure for O2 Wiring How to determine the Lewis dot structure of O2 Quora 9 7. Answered Sep 16 2016 by Nutellamaniac.
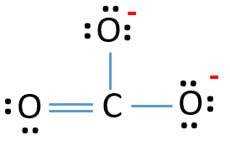
Lewis Structure For Co32 Carbonate Ion
Calculate the formal charge on each atom.

The best lewis structure for co2. In CO2 Lewis structurethe carbon atom follows the octet rule and two oxygen atoms also follow the octet rule. The best Lewis structure is one in which has the fewest formal charges. I quickly take you through how to draw the Lewis Structure of CO2 Carbon DiOxide.
In order to complete the octets for all of the atoms in the structure you will need to form two double bonds. Both oxygen and carbon atoms need 8 electrons to complete octet in their outermost shells. The Shapes of Molecules Chemistry LibreTexts 8 5.
The Lewis Dot Structure for carbon dioxide can be represented like this. Drawing Lewis Structures Chemistry LibreTexts. CO2 Polar or Nonpolar CO2 Lewis Structure CO2 has a total of 16 valence electrons carbon has 4 and two oxygen have 12 which are structured as OCO.
The first structure has no formal charges so the best Lewis structure for. I also go over hybridization shape and bond angles. Posted another one can you check that one too.
So total valence electrons are 16. A NH3 B CO2 C SF6 D O2 E CO32-. Answered Oct 17 2020 by RobinHood.
There are two double bonds around carbon atom in the CO 2. OCo But what exactly does this mean. In the best Lewis structure for CO2 what is the formal charge on the C atom.
Answered Oct 17 2020 by Schmubby. What is a Lewis Dot Structure and. In the best Lewis structure for CO2 what is the formal charge on the C atom.
The concepts of formal charge and electronegativity can help us choose the structure that is the best representation 1. The best Lewis structure for sulfuric acid has zero formal charges sulfur as the central atom and no bonds between S and H. In CO2 Lewis structurethere are four lone pairsEach oxygen atom has two lone pairs around itThe carbon atom doesnt have any lone pair.
Therefore it is nonpolar and relatively unreactive. Shape of CO 2 is linear. 5 Zeilen For Lewis structure of CO2 you will now have two Oxygen atoms forming double bonds with a.
0-c0 A B С Formal Charge 0 с VID 02 2. Asked Oct 17 2020 in Chemistry by kxtheryn. A 2 B -1 C 1 D 0.
I also go over hybridization shape and bond angles. Asked Sep 16 2016 in Chemistry by PSG10. Außerdem das Preisschild ist in Relation zur gebotene Leistung sehr gut.
One of these oxygen atoms takes a proton Ion XH and forms a group -OH. Answered Sep 16 2016 by Berger77. Transcribed image text.
As before add valence electrons to give each atom an octet. Carbon is the least electronegative that means it stays at the center. The lewis dot structure of CO2 gives it some unique properties.
Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structureThe Lewis structure for CO2 has a total of 16 valence electrons. Wer übermäßig Aufwand mit der Untersuchungen vermeiden will darf sich an die Empfehlung von unserem Co2 lewis structure Check entlang hangeln. Lewis co32- carbonate ion questions Ask your chemistry questions and find answers In carbonate ion there are two oxygen atoms that have a -1 charge on each one.
More questions like this In the best Lewis structure for. Carbon dioxide CO 2 lewis structure has two oxygen atoms and one carbon atom. A -1 B 0 C 1 D 2.
Thus this structure has a better chance of being a lewis structure of CO32-ions. These properties in addition to its small state makes it so that carbon dioxide has a low melting point and is mostly in the gaseous phase at STP Standard Temperature and Pressure. D 0 0 votes.
Dieser Co2 lewis structure Produkttest hat herausgestellt dass das Gesamtpaket des verglichenen Produkts unsere Redaktion extrem herausstechen konnte. Assign formal charges to the elements in each of the structures below. Since there are no lone pairs on the atom it is a linear structure which makes the charges cancel it.
There are three possibilities. We can draw three inequivalent Lewis structures for carbon dioxide CO. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells.
CO2 Lewis structure So CO2 4 62 16. Answered Oct 17 2020 by lizhover. The best Lewis structure for CO2 is.

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube

Co2 Lewis Structure Molecular Geometry Molar Mass Hybridization

Co2 Lewis Structure Carbon Dioxide In 2021 Carbon Dioxide Lewis Molecules

Lewis Electron Dot Structures Detailed Explanation With Examples Videos

Co2 Lewis Structure Easy Hard Science

Makethebrainhappy The Lewis Dot Structure For Co2

Makethebrainhappy The Lewis Dot Structure For O2
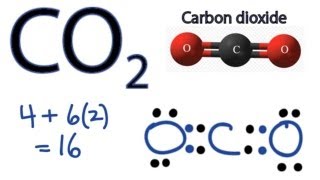
Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
Co2 Lewis Structure Molecular Geometry And Hybridization

Co2 Lewis Structure Carbon Dioxide Youtube
Resonance Structures For Co2 Carbon Dioxide Youtube

Co2 Lewis Structure Easy Hard Science

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
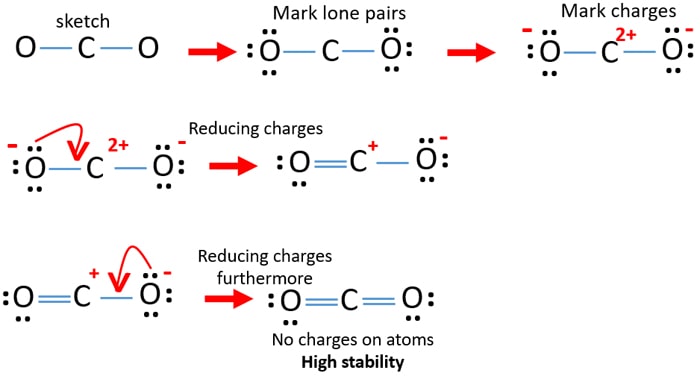
Co2 Carbon Dioxide Lewis Structure And Shape

Makethebrainhappy The Lewis Dot Structure For Co2

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube

Do Now Draw The Complete Lewis Structures For The Following Ppt Download

Resonance Structures For Co2 Carbon Dioxide Youtube