Draw The Lewis Dot Structure For C2h4
Is it polar or nonpolar. Draw the Lewis structure for ethylene C2H4.
Lewis Electron Dot Structures Ck 12 Foundation
Please forgive my very poor drawing skills.
Draw the lewis dot structure for c2h4. Calculate the total valence electrons in the molecule. Step-by-step tutorial for drawing the Lewis Structure for C2H4. Electron Dot Structure for ethene C2H4.
Lewis benzene dot 3. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. C2h4 Lewis Dot Structure - How to draw the lewis structure for c2h4.
You should consult the lewis structure rules and a periodic table while doing this exercise. Please answer the following questions here. According to the VSEPR chart the shape of the ethene molecule is trigonal planar.
All electrons are shared within bonds in this compound. Draw the electron-dot structure for C2H4. The key to understanding how t.
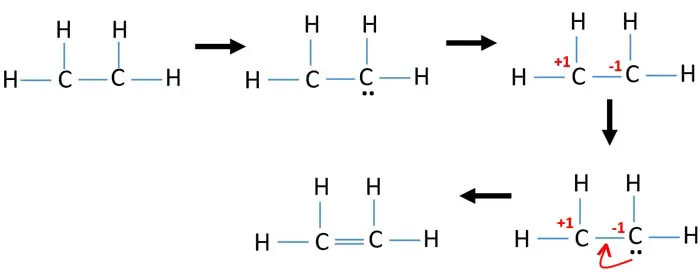
Include all lone pairs of electrons. Its C2H4 and we want to write the dot structures for ethene. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them.
Alternatively a dot method can be used to draw the lewis structure. Total valence electrons available for ethene C2H4 lewis structure 42 14 12 valence electrons C2H4 has two carbon and 4 hydrogen atom 2. A Arrange the molecules in the increasing order of their carbon-carbon bond strength.
Electron Dot Structure for ethane C2H4. Lewis Structures for C2H4. C2H Draw the Lewis dot structure for C2H4.
5 c2h4 yet more lewis structures answers. Lewis Structures for C2H4. It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
H O N S P F Br Part D C2N2 Draw the Lewis dot structure for C2N2. By signing up youll get thousands of. Draw Lewis Dot structures for C2H6 C2H2 and C2H4.
Ortho-dichlorobenzene C6H4Cl2 is obtained when two of the adjacent hydrogen atoms in benzene are replaced with Cl atoms. Include all lone pairs of electrons. When there are no polar bonds in a molecule there is no permanent charge difference between one part of the molecule and another and a molecule can possess polar bonds and still be nonpolar.
It has 1 valence electron. This means that the carbon atoms share 4 electrons. In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
Use information from step 4 and 5 to draw the lewis structure. Carbon is in group 4 sometimes written 14 so it has 4 valence electrons. To draw the c2h4 lewis.
Lewis dot structure of C 2 H 4. Such as polarity and bond length we will draw the lewis or electron dot structure. Transcribed Image Textfrom this Question.
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Showing top 8 worksheets in the category chemical bonds lewis dot structures answers. Find the least electronegative atom and placed it at center.
C H O N S P F Br Х More Part C HCO3 Draw the Lewis dot structure for H2CO3. The electron dot structure is drawn using Lewis-dot structure. The key to understanding how to distribute the valence electrons is to recognize the need for a double.
If we come way over here to Hydrogen its in group 1. A step-by-step explanation of how to write the Lewis Dot Structure for C2H4 ethene. What is its molecular shape.
Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure. Lets take a look. Carbon has 4 and hydrogen has 1 making a grand total of 12 e- 42 41.
It consists of two carbon molecules and 4 hydrogen molecules. To do that we always count our valence electrons up first. To draw the Lewis structure for C2H4 the total number of valence electrons must be known.
Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond. 10 sf6 lewis structures practice worksheet solutions. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure EtheneFor the C2H4 structure use the periodic table to find the total number of val.
There are two triangles overlapping each other as we can see in the diagram.

Write The Electron Dot Structure Of Ethene Molecule C2h4

Write The Electron Dot Structure Of Ethene Molecule C2h4 Delhi 2011 Brainly In

Draw The Lewis Structure For Ethylene C2h Clutch Prep

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
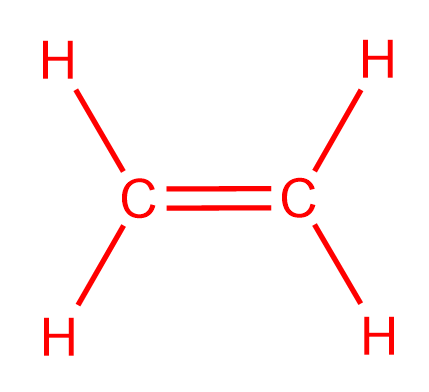
Draw The Lewis Structure For The C2h4 Ske Clutch Prep
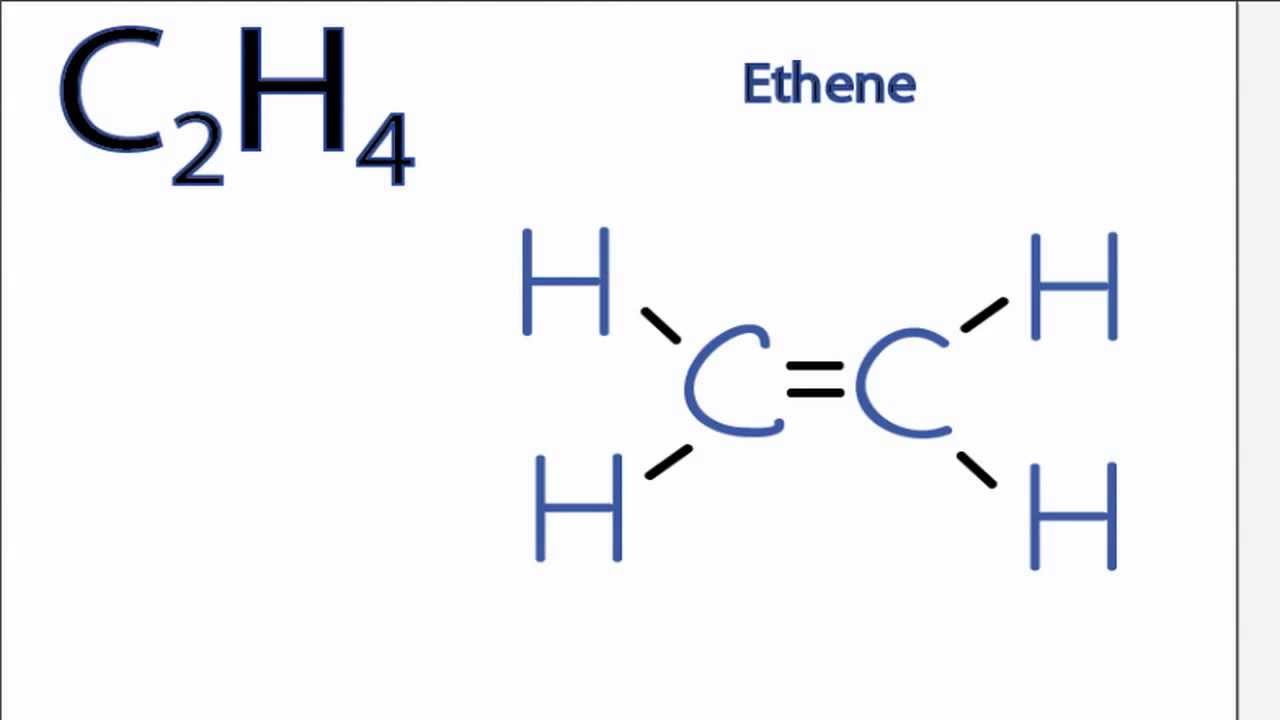
Is C2h4 Polar Or Nonpolar Youtube
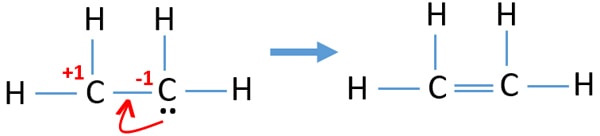
Ethene C2h4 Lewis Structure Hybridization

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

Ethene C2h4 Lewis Structure Hybridization

C2h4 Molecular Geometry Shape And Bond Angles Youtube
Lewis Structure Of C2h4 Biochemhelp
![]()
Draw And Explain The Lewis Structure Of C2h4 Study Com
Lewis Electron Dot Structures Ck 12 Foundation

C2h4 Lewis Structure C2h4 Lewis Structure Molecular Geometry

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Draw The Lewis Structure For The C2h4 Ske Clutch Prep

