Clo3- Lewis Structure Octet Rule
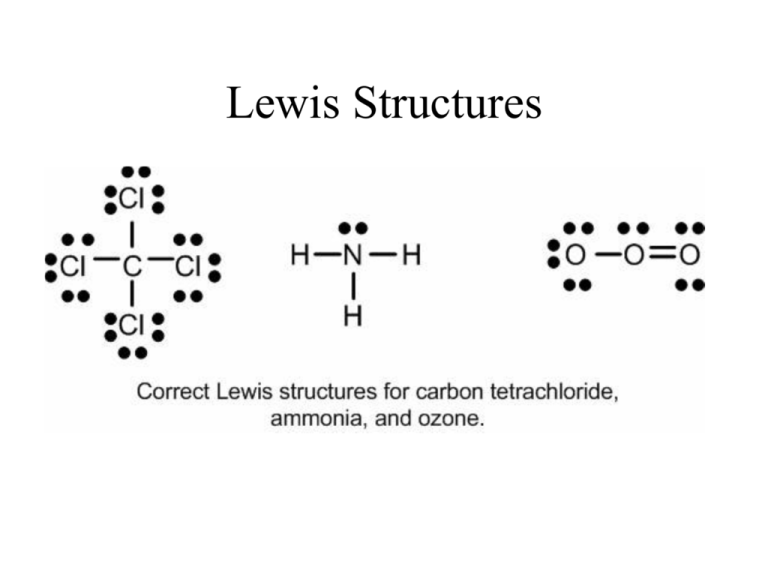
70 More Lewis Dot Structures Chlorine does not follow the octet rule. The chemical formula ClO 3 represents Chlorate ion.

Draw A Lewis Structure That Obeys The Octet Rule For Each Of Clutch Prep
All other atoms do not have charges.
Clo3- lewis structure octet rule. In each case the first atom lis. Here are the bonds predicted by Lewis rules. Does ClO3 obey the octet rule.
As you can see from the structure above B has only 6 valence electrons. Steps for Writing Lewis Structures. A dash or line is usually used to indicate a shared pair of electrons.
2 count all valence electrons. Elements in the first 2 periods of the Periodic Table do. How many charges in atoms of chlorate ion lewis structure.
Lewis Dot of the Chlorate Ion. ClO3-ClO4-NO3-NH4 Any help at all on how to solve these would be appreciated. 4 if there are any electrons left distribute them as lone electron pairs to maximize the number of the octets.
Why does the chlorine in ClO2- lewis structure contains electrons of more than 8 and violate the octet rule. You will learn all steps and rules of lewis structure drawing. It will hold more than 8 electrons.
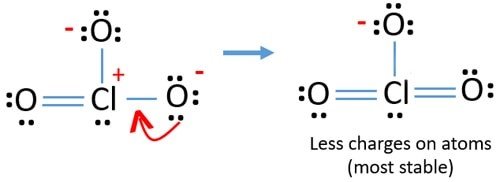
Write a Lewis structure that obeys the octet rule for each of the following ions. 5 if some of the atoms lack octet structures change as many. In this case as seen in the figure Chlorates exist in a 5 oxidation state.
Chlorine having valence electrons in the 3rd energy level will also have access to the 3d sublevel thus allowing for more than 8 electrons. It will hold more than 8 electrons. Assign formal charges to each atom.
Lewis Structure or electron-dot structure is one such representation of chemical bonding between atoms inside a. 1 choose a center atomdraw the molecular skeleton. Lewis structure with formal charges.
Therefore shape of ion is trigonal pyramidal. Write a Lewis structure that obeys the octet rule for each of the following ions. Chlorine having valence electrons in the 3rd energy level will also have access to the 3d sublevel thus.
Lewis Structure of ClO4. It will hold more than 8 electrons. A step-by-step explanation of how to draw the ClO3- Lewis Structure Chlorate Ion.
Hydrazine N2H4 is a good reducing agent that has been used as a component in rocket fuel. The correct formula of chlorate ion is ClO 3-. This chemistry video tutorial explains how to draw the lewis structure of the Chlorate ion ClO3-My Website.
Draw the lewis structure for chlorate ClO3-. How some atoms come together and form covalent bonds with each other inside a molecule is something we need to visualize to be able to grasp the science behind this. Chlorine can reach oxidation states of 1 3 5 and 7.
Now we are going to learn how to draw the lewis structure of ClO 4-ion step by step. Assign formal charges to each atom ClO3-ClO4-NO3- and NH4. However Lewis rules predict that one of the bonds between Boron and chlorine should be a double bond in order for B to have an octet.
The ClO3- Lewis structure is a good structure to help you understand w. Whenever the d-orbitals and beyond to it participate in bonding with other atoms then an expanded octet is produced. 3 draw a single bond from each surrounding atom to the central atom subtract 2 valence electrons for each bond.
Lewis Structure of perchlorate ion. There are three σ bonds and a one lone pair around chlorine atom in lewis structure of ClO3- ion. For this structure give each atom an octet and do not include a formal charge.
Chemical bonding is one of the most important and interesting chapters of chemistry. A step-by-step explanation of how to draw the ClO3- Lewis Structure Chlorate Ion. In the Lewis model a single shared pair of electrons constitutes a single bond.
70 More Lewis Dot Structures. Examples of stable odd-electron molecules are NO NOAlthough the O atom has an octet of electrons the N atom has only seven electrons in its valence shell. Chlorine does not follow the octet rule.
There is no molecule or ion as ClO3. Only one oxygen atom has a -1 charge. Draw Lewis structures step by step.
There are four oxygen atoms and chlorine atom in perchlorate ion. ClO3- Lewis Structure Molecular Geometry Hybridization Shape. Write the Lewis structure that obeys the octet rule for each of the following molecules and ions.
Chlorine atom in ClO2- lewis structure expanded the octet because it has d-orbitals in the third principal energy level hence it has extra orbitald-orbital for. Therefore overall charge of chlorate ion is -1. Lewis Structure of ClO 3- Chlorate ion Lewis structure of ClO 3- ion is drawn step by step in this tutorial.
An octet is a set of eight - think octopus an animal with eight legs octagon a figure with eight sides and octomom a woman who had eight babies at one time. Lewis Dot of the Chlorate Ion ClO3- Chlorine does not follow the octet rule. A well-known example is BFThe third violation to the octet.
Determine the total number of valence electrons in the molecule or ion. COVID-19 is an emerging rapidly evolving situation. In chemistry the octet.
Get the detailed answer. With an abundance of oxidizing elements the Chlorate ion and its salts make for powerful. Lewis structure of ClO3- ion is drawn step by step in this tutorial.
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms.

Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula
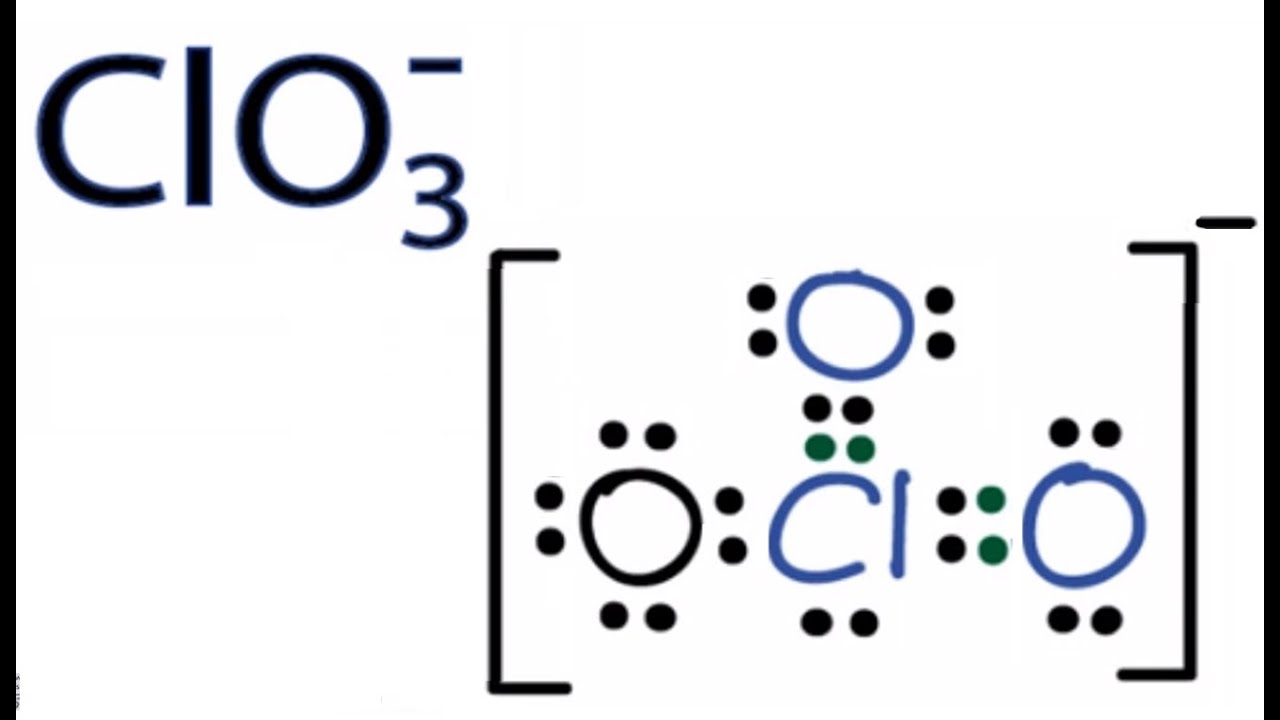
Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube
Clo3 Lewis Structure Molecular Geometry Hybridization Shape

How To Draw The Lewis Structure Of No3 Nitrate Ion Youtube

Lewis Structure Of Clo3 Chlorate Anion Youtube High School Chemistry High School Chemistry

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas

Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula
Https Learning Hccs Edu Faculty Paul Bradley Chem1411 Other Course Handouts Examples Of Formal Charge And Lewis Dot Structures

Using The Octet Rule Figure Out And Draw The Lewis Structure For Bromate Chlorate Study Com

126 Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube Chemistry Classroom Science Chemistry Chemistry
What Is The Lewis Dot Structure Of Co3 2 Quora

Clo3 Lewis Structure Molecular Geometry Hybridization Shape

If3 Lewis Structure How To Draw The Lewis Structure For If3 Youtube
Https Learning Hccs Edu Faculty Paul Bradley Chem1411 Other Course Handouts Examples Of Formal Charge And Lewis Dot Structures
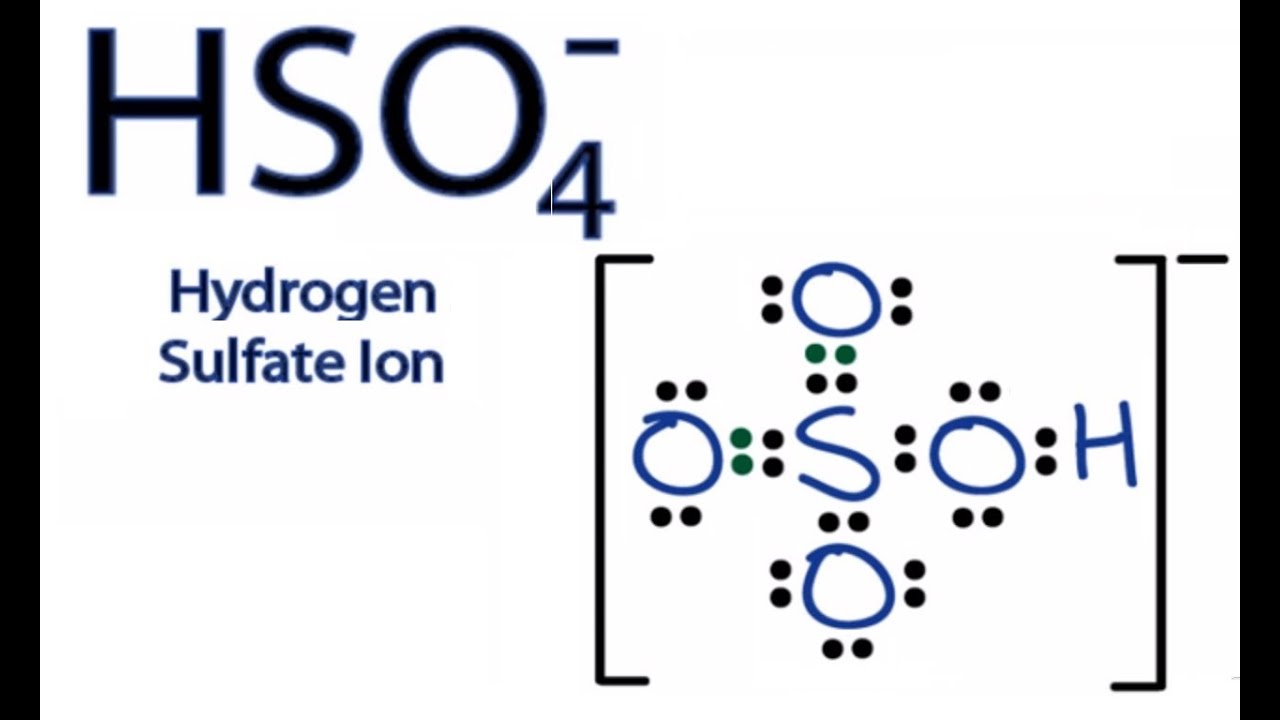
Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube

Clo3 Lewis Structure Molecular Geometry Hybridization Shape

Nh4 Lewis Structure How To Draw The Dot Structure For Nh4 Ammonium Science Chemistry Molecular Geometry Chemistry