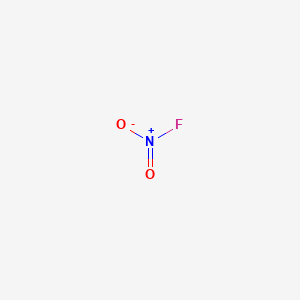
Lewis Structure For Fno2
If more than one Lewis structure can be drawn use formal charges to decide on the most. Because of this well try to get as close to an octet as we can on the central Nitrogen N atom.

Molecular Structure And Polarity Chemistry For Majors
Nitrogen dioxide does not have a single Lewis structure on account of its relatively strange electron configuration.
Lewis structure for fno2. The location of the double bond changes over time meaning that at any point either of the oxygen atoms could have a double bond with the nitrogen atom. Draw An Acceptable Resonance Lewis Structure For FNO2 Showing Any Formal Charges And Select The Atoms Where You Would Expect Delocalized Molecular Orbitals To Form. The Fluorine And Oxygen Atoms Are Bonded To The Nitrogen Atom.
Resonance In this lesson well review Lewis dot structures and how to draw them. NO2F - Nitryl Fluoride. Lewis Structure is a diagrammatic representation of any given molecule with the help of the constituent atoms and the position and arrangement of electrons to form bonds and lone pairs.
Include any resonance structures. The molecular geometry of NO 2 F is trigonal planar with asymmetric charge distribution on the central atom. Nitrogen N is the least electronegative atom and goes at the center of the NO 2 F Lewis structure.
Three single bonds zero double bond and eight lone pairs. Draw the Lewis structure for FNO and determine the number of bonds and lone pairs. This is a limited theory on chemical bonding nature and electronic structure but provides a simple viewpoint towards the formation of any molecular composition.
Then learn about resonance and resonance structures for molecules and polyatomic ions. Be sure to put brackets along with a negative sign around the NO 2- Lewis structure when you are done to show that it is an ion with a negative charge. This will mean that it will only have 7 valence electrons.
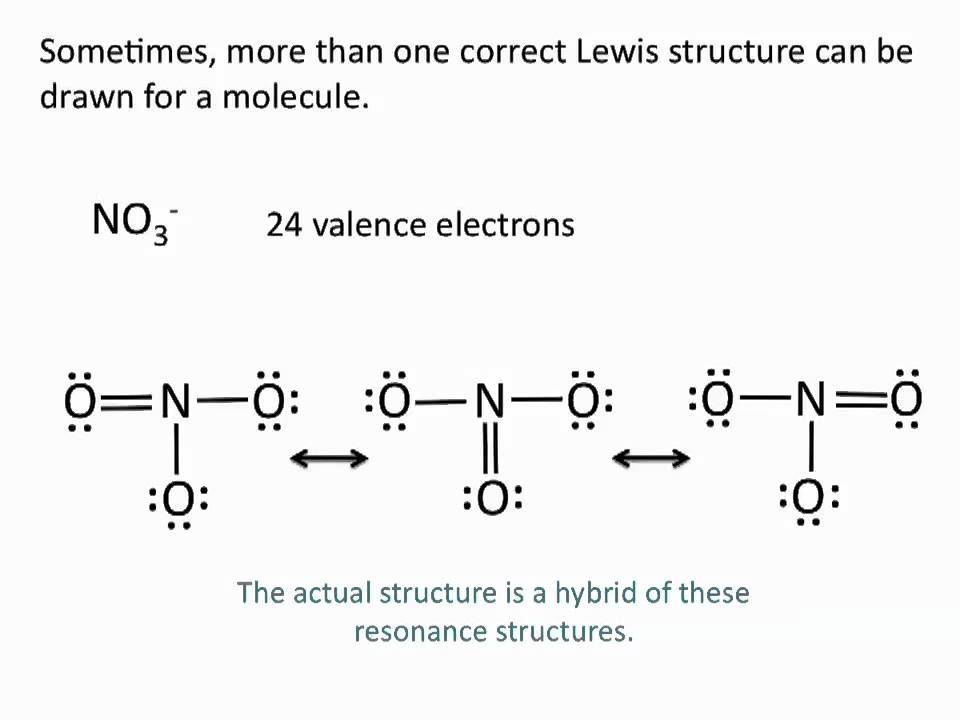
NO 2- has a total of 18 valence electrons. Three single bonds no double bonds and no lone pair. Two single bonds two double bonds and no lone pairs.
It is a molecular species not ionic consistent with its low boiling point. There are a total of 24 valence electrons in the NO 2 F Lewis structure. With NO 2 F youll need to form a double bond between one of the Oxygen atoms and a Nitrogen atom to fill the octets and still use only the 24 valence electrons available for the molecule.
The NO2 Lewis structure has a total of 17 valence electrons. Get the free Lewis structure widget for your website blog Wordpress Blogger or iGoogle. Nitrvl Fluoride FNO2 Is Veiy Reactive Chemically.
Find more Chemistry widgets in WolframAlpha. In the Lewis structure for NO2 the Nitrogen atom is the least electronegative atom and goes at the center of the. Two single bonds one double bond and eight lone pairs.
A step-by-step explanation of how to draw the NOF2 Lewis Dot Structure Nitryl fluoride For the NOF2 structure use the periodic table to find the total num. Then draw the 3D molecular structure using VSEPR rules. A step-by-step explanation of how to draw the PO3 3- Lewis Dot Structure Phosphite ionFor the PO3 3- structure use the periodic table to find the total nu.
A FNO2 B XeF_5 Draw Lewis structures for the formula above. Its not common to have an odd number of valence electrons in a Lewis structure. Nitryl fluoride NO 2 F is a colourless gas and strong oxidizing agent which is used as a fluorinating agent and has been proposed as an oxidiser in rocket propellant s though never flown.
Nitryl Fluoride NO2F Molecular Geometry Polarity. A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure Nitrite ionFor the NO2 - structure use the periodic table to find the total number. This problem has been solved.
First draw the Lewis dot structure. The Lewis structure for NO 2- Nitrite Ion comes up quite often in chemistry. 6 rows Nitryl fluoride FNO2 CID 66203 - structure chemical names physical and chemical.
As such nitrogen dioxide is represented by the resonance Lewis structure.
7 E Chemical Bonding And Molecular Geometry Exercises Chemistry Libretexts

No2cl Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
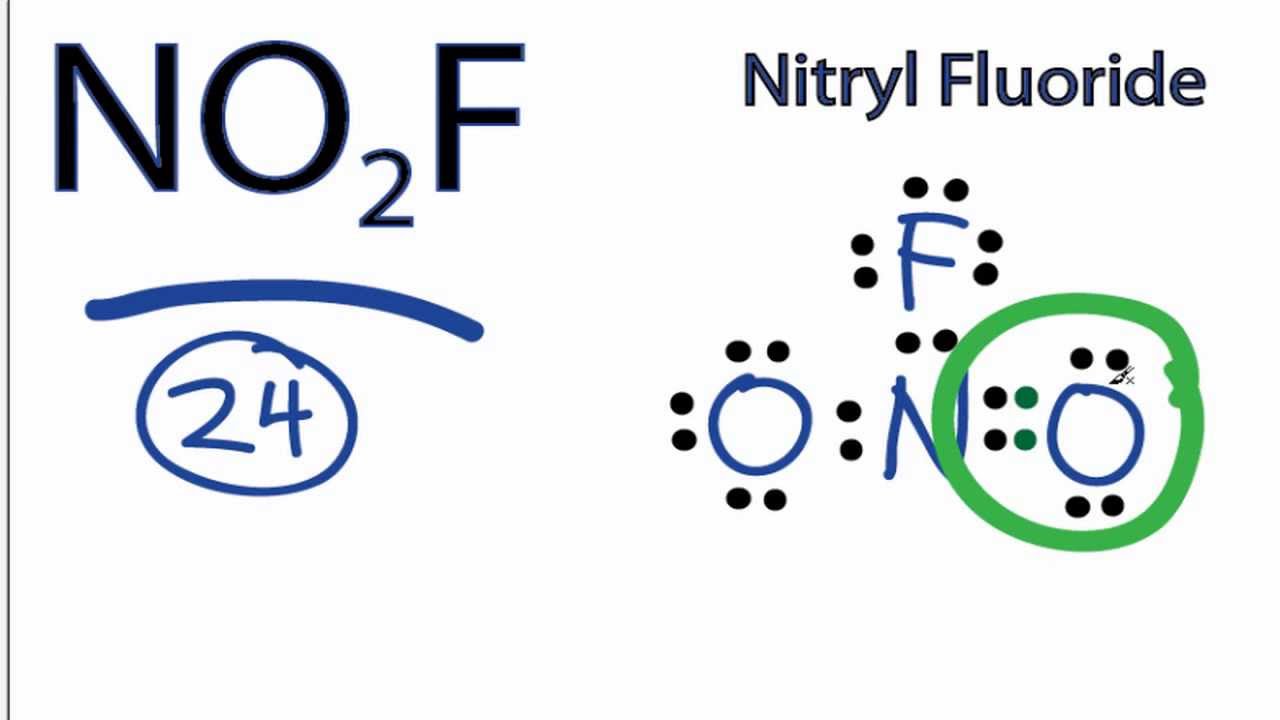
No2f Lewis Structure How To Draw The Lewis Structure For No2f Youtube
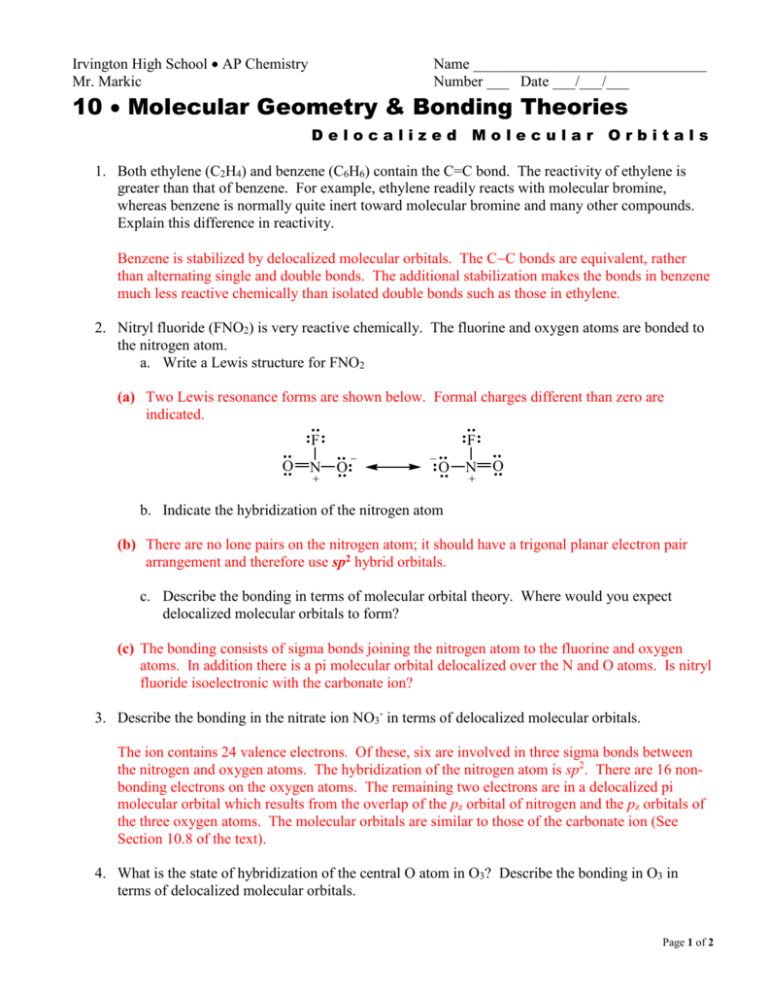
Irvington High School Ap Chemistry

No2cl Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

No2cl Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Add Lone Pairs To These Lewis Structures O Clutch Prep

Lewis Dot Structure No2f Youtube

No2f Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

A Fno2 B Xef 5 Draw Lewis Structures For The Formula Above Include Any Resonance Structures If More Than One Lewis Structure Can Be Drawn Use Formal Charges To Decide On The Most
7 E Chemical Bonding And Molecular Geometry Exercises Chemistry Libretexts

No2f Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Lewis Dot Structure Calculator Novoeasysite

Add Lone Pairs To These Lewis Structures O Clutch Prep

Nitryl Fluoride Structure Fno2 Over 100 Million Chemical Compounds Mol Instincts
7 E Chemical Bonding And Molecular Geometry Exercises Chemistry Libretexts

A Fno2 B Xef 5 Draw Lewis Structures For The Formula Above Include Any Resonance Structures If More Than One Lewis Structure Can Be Drawn Use Formal Charges To Decide On The Most