Valence Electrons In Ni3
Group 8 - 3 charge d 5 or 3d 5 8 - 3 5. Compound formation of nitrogen by valence electrons.
Ni3 Molecular Geometry Bond Angles Electron Geometry Youtube
Iron is in group 8 so.
Valence electrons in ni3. We first need to find the number of. Hence the electrons present in the valence shell possess the highest energy compared to the electrons present in the inner orbits. The same procedure can be applied to any transition metal complex.
Valence electrons in simple words are the electrons revolving continuously in the outermost shell or orbit of an atom. Nickel has eight electrons in the 3d orbital and two electrons in the 4s orbital which means nickel has 10 total valence electrons. As a result they.
It has a negative charge now once we get to the charge of the I3-ion therefore the amount of this charge would be 1. At the very beginning we need to calculate the total valence. To find out the Lewis structure of I3 let us check all the steps in the following process.
The electron-dot structure of NH 3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This is because transition metals most of the metals in the middle of the periodic table have electrons in both the s shell and the d shell. Also known as Lewis electron dot structures it is based upon the octet rule of valence electrons.
We know that the valence electrons in nitrogen are. Valence electrons are the electrons in the highest occupied principal energy level of an atom. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron.
For example consider the. The iodine atoms valence electrons are 7 since there are seven electrons within the outermost shell. The reason it has 10 is because nickel is a transition metal so the d and s electrons can participate in chemical bonding.
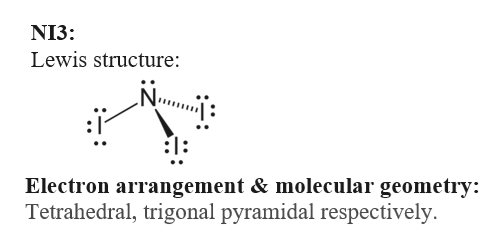
It is a key concept to understand the characteristics and hybridization of a molecule since it helps us draw the chemical structure. NI3 is called Triiodoamine or nitrogen triiodide. Since the last shell of a nitrogen ion has 8 electrons the valence electron of nitrogen ion is eight.
Thus the number of valence electrons that it may have depends on the electron configuration in a. This shell is the farthest from the nucleus. The first is to use the Periodic Table to figure out how many electrons Boron has in.
And tilting it with respect to the detector. That is in this case the valence of nitrogen ions is 3. This is the Valence Shell Electron.
Structure of the octahedral ferricyanide anion. We just add the valence electrons of each atoms involved. There are two ways to find the number of valence electrons in Boron B.
For I3- well end up with 6 additional valence electrons after filling the octets on the outside iodine atoms. Once we know how many valence electrons there are in I3- we can distribute them around the central atom and attempt to fill the outer shells of each atom. Angle-resolved photoemis-sion intensities for different photoelectron emission geom-etries were recorded by rotating the sample around its sur- face normal azimuthal fscan.
Now consistent with the formula position all the values. Nitrogen participates in the formation of bonds through its valence electrons. Valence electrons are the electrons of an atom that can participate in chemical bonding.
Likewise what is the electron geometry of i3. Because the overall charge of the complex is 3- Fe is in the 3 oxidation state and its electron count is 3d 5. For a main-group element the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n.
To write the configuration for the Nickel ions first we need to write the electron configuration for just Nickel Ni. How many valence electrons. The rule is to count all of irons valence electrons as d-electrons.
Valence band electrons were detected with a fixed hemispherical analyzer at a typi-cal photon energy of 100 eV energy resolution of 55 meV and angular resolution of 14. Since two out of three iodines are monovalent the monovalent atom number is 2. Nickel has eight electrons in the 3d orbital and two electrons in the 4s orbital which means nickel has 10 total valence electrons.
For the NI 3 Lewis structure there are a total of 26 valence electrons available. For nickel specifically there are eight electrons in the 3d shell and two in the 4s shell. NI 3 is very similar to NH 3 and NF 3.
Beryllium has two valence electrons. First we need to count the total number of valence electrons. Valency of nickel 10.
The electrons that determine valence how an atom reacts chemically are those with the highest energy. The reason it has 10 is because nickel is a transition metal so the d and s electrons can participate in chemical bonding. I 7 x 3-----.
The outermost shell or the valence shell is the shell having the highest energy. For the Lewis structure for I3- you have. 7 1 2 2 22 1022 5.
Well just put these on the central atom since thats the only place left for them to go. In the second period elements listed above the two electrons in the 1 s sublevel are called inner-shell electrons and are not involved directly in the elements reactivity or in the formation of compounds. Valence electrons are the electrons of an atom that can participate in chemical bonding.
Read more on it here. The molecular geometry of I3 triiodide is linearA triiodide ion is negatively charged and is formed from the bonding of three iodine atomsThere are seven electrons in the outermost shell of iodine which can readily react with other chemical species to form bonds. Thus the Electron Group geometry of each central atom in a structure can be determined by simply counting the number of groups of electrons around the atom then considering how those groups would arrange themselves to be as far apart as possible.
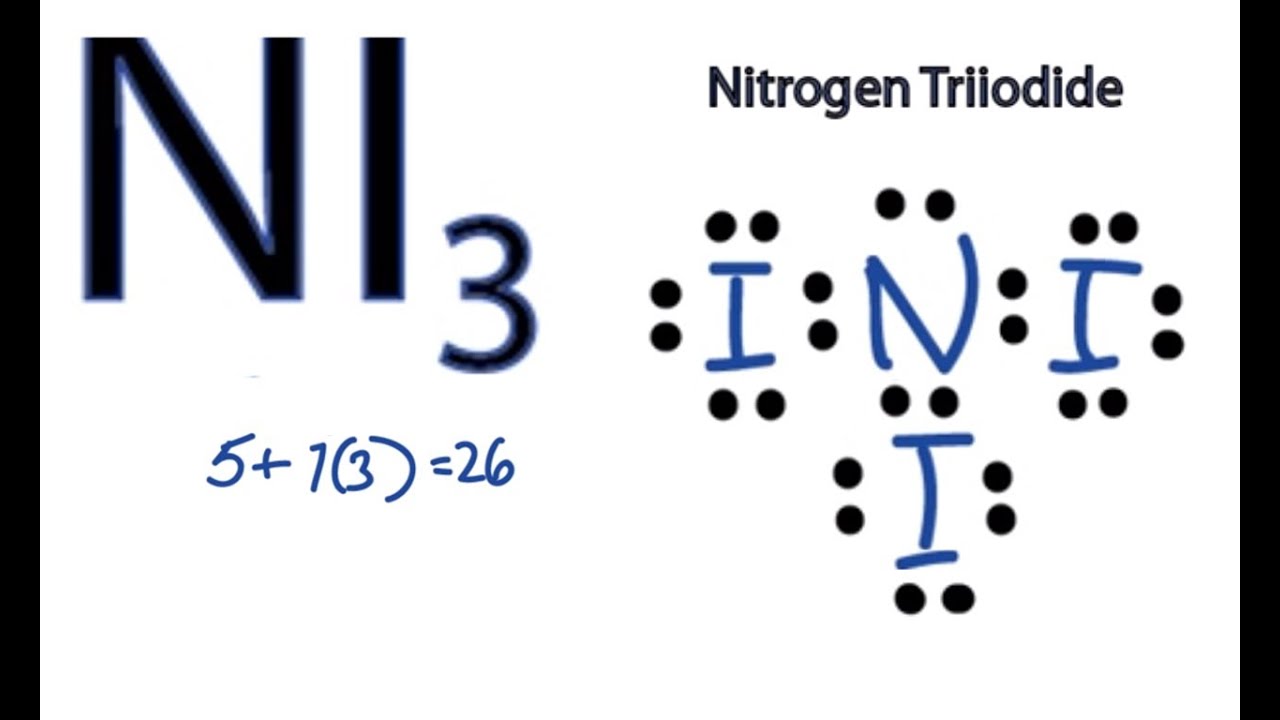
Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

Electron Configuration For Ni Ni2 And Ni3 Nickel And Nickel Ions Youtube

What Is The Lewis Structure Of Ni3 Study Com
Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Chapter 3 Molecular Shape And Structure Flip Ebook Pages 1 11 Anyflip Anyflip

Band Structure Of Ni 3 Nb In The Do A Structure Download Scientific Diagram
When A Covalent Lewis Structure Is Drawing Using One Nitrogen N How Many Lodine Atoms Will Bond To The Nitrogen Quora

10 This Questions Below Deal With Structure Of Ni3 Chegg Com

Chapters 4 5 Chemical Bonding Valence Electrons Outermost

Molecular Models Activity Ppt Download
9 Co2 Total Number Of Valence Electrons Edg Model Chegg Com
Solved Draw The Lewis Structure For Ni3 How Many Valence Chegg Com

How To Draw Lewis Structure Of Ni3 Drawing Easy

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

Table For Answering Lewis Structures And Vsepr Theory Practice

Answered Draw The Lewis Structure And Determine Bartleby

Ni3 Ipr2im 3 µ2 Co 3 µ3 Co A Co Stabilized Nhc Analogue Of The Parent Neutral Nickel Chini Type Cluster Berthel 2019 European Journal Of Inorganic Chemistry Wiley Online Library