Brf5 Lewis Structure Bond Angles
Quiz your students on Lewis Structure For BrF5 Molecular Geometry Bond Angle Hybridization Polar or Nonpolar using our fun classroom quiz game Quizalize and personalize your teaching. The molecule has a central bromine atom surrounded by five fluorides and a pair of electrons.
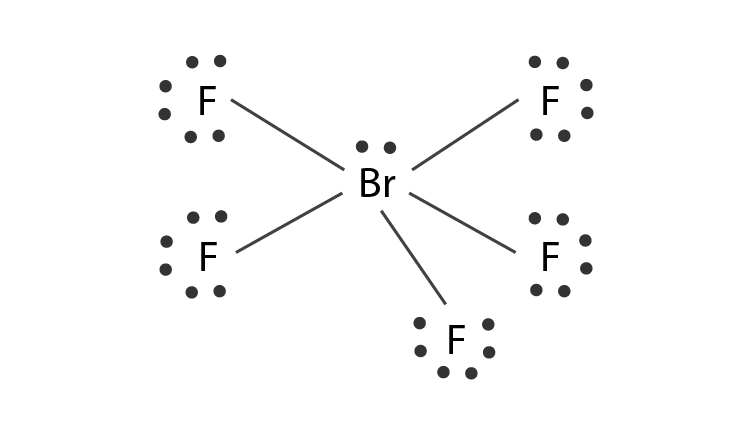
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5
These pairs lie along the equator of the molecule.
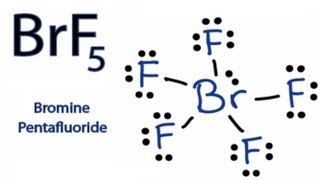
Brf5 lewis structure bond angles. BrF5 Bond Angles. Quiz your students on BrF4 Lewis Structure - Bond Angle Hybridization Polar or Nonpolar Molecular Geometry using our fun classroom quiz game Quizalize and personalize your teaching. Draw the Lewis Structure and use that to fill in the following chart.
C is the Best Lewis structure for OCl 2 with 1109 o bond angle. Check out the below picture to clarify your confusion further. From the Lewis structure it can be observed that Bromine has an expanded octet.
Geometrics atom NH3 CH4 BrF5 BrF3 CO32 CO2 H2O IC2- ICI4-. This can be studied with the help of Valence Shell Electron Pair Repulsion VSEPR theory which says the overall shape of a molecule is decided by the total number of bonding and non-bonding electrons along with their orientation around the central atom. NO2 Molecular Geometry Shape and Bond Angles Note.
Please show me how to complete this chart. The bond angle of BrF5 is 90º. The Molecular geometry of NCl 3 is Trigonal pyramidal Fig1.
Lone pairs are found in one of the hybrid orbitals. If we look at the alphabet T which this molecule resembles we can infer that the angle has to be 90 degrees. The constituent atoms repel each other in accordance with the VSEPR theory.
Hybridization Number of o and a Bonds CIF3 chlorine trifluoride XeF2 SF6 BrF5. Brf5 atom bonding pairs non many central electrons lewis structure brf study elements since both. Bromine pentafluoride BrF5 lewis dot structure molecular geometry polar or non-polar bond angle Step-1.
Molecular formula Lewis structure Electron-group arrangement AXE formula Bond angle Molecular shape Polar or nonpolar. The bond angles in BrF 5 are 90. Hybridization Number of o and a Bonds CIF3 chlorine trifluoride XeF2 SF6 BrF5 XeF4.
2 The structure of BrF5 is square pyramidal. Steric Number s Overall Electron Pair Hybridization of the central Formula Lewis Structure Bond Angle s Shape Net Dipole. The first step is to.
Rest two pairs lie perpendicular to the equatorial axis known as the axial pairs. BrF 5 Molecular Geometry And Bond Angles BrF 5 molecular geometry is said to be square pyramidal with a bond angle of 90 o each. The angle between the three pairs lying on the central position is 120 degrees and the angle between the axial and equatorial position is 90 degrees.
N 2 O NO 2- NCl 3. Determine the total number of outermost valence shell electrons in the BrF5 molecule. BrF5 Number of Valence e Whole Structure Areas of e Density Around Central Atom.
Bromine Pentafluoride BrF5 is a polar molecule because the molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution and with a bong angle of 90. Structures of Molecule Names. Molecular Geometry Select an answer Bond Angles Central Atom This problem has been solved.
But here the bond angles are reduced to around 86 degrees which is. To determine the molecular geometry for Bromine Pentafluoride we go back to its Lewis structure. But Due to trivial distortion however the bond angles FBrF are slightly less than 90º 85º.
Non-Bonding Areas Central Atom. The bond angle between each participating atom is 90 where the structure of BrF5 is square pyramidal. BrF5 Molecular Geometry and Shape.
The number of FBrF angles having the value of 90º is eight 8. Brf5 structure vsepr chemistry electron brf valence pair repulsion theory shell. The central atom bromine forms 5 sigma bonds with fluorine atoms.
BrF5 lewis dot structure has 10 sharing electrons and 32 non-sharing electrons. Locate the atom with the least electronegative charge and place it in. Select an answer Bonding Areas Central Atom.
What is the molecular geometry of NCl3. Structure lewis brf5 xef2 draw theory using shapes predict vsper answers hi given. Place the following in order of decreasing X-A-X bond angle where A represents the central atom and X represents the outer atoms in each molecule.

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Square Pyramidal Villanova College Chemistry Blog

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
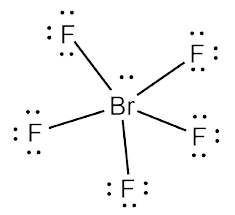
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
What Type Of Hybridized Orbital Is Used By The Central Atom Of Brf5 Quora
Bromine Pentafluoride Brf5 Lewis Dot Structure Youtube
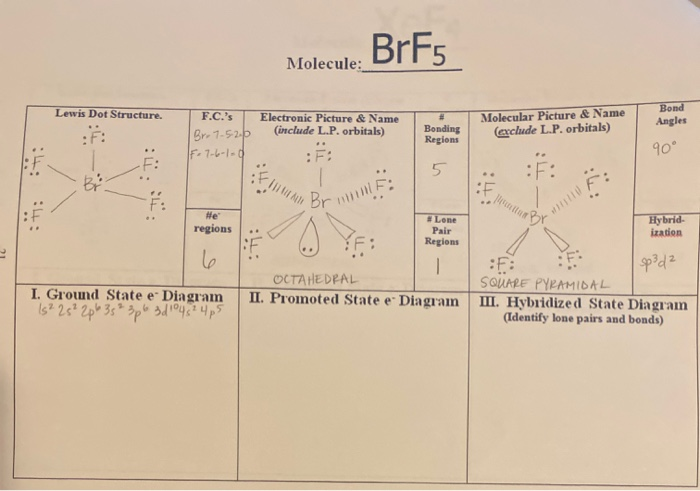
Brf5 El Brillill Molecule Lewis Dot Structure Chegg Com

Bromine Pentafluoride Brf5 Is Sometimes Clutch Prep

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
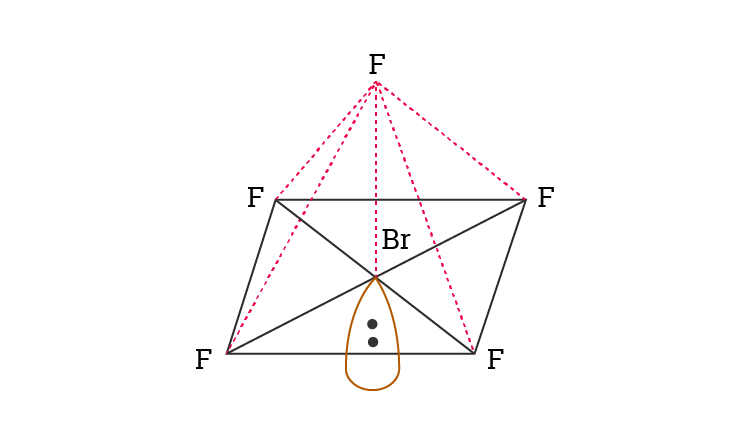
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5

Bromine Pentafluoride Brf5 Is Sometimes Clutch Prep
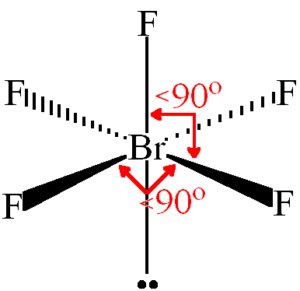
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Bond Angles In Brf5 Chemistry Stack Exchange
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape