Is H20 Polar Or Nonpolar Molecule
H20 polar or nonpolar. Water is a Polar Covalent Molecule Water H2O like hydrogen fluoride HF is a polar covalent molecule.
Polar And Non Polar Molecules Vce Chemistry
Every molecule has a polarity.
Is h20 polar or nonpolar molecule. Yes water H2O is polar. Is h20 polar or nonpolar. TASK ANSWER 1 Sort by.
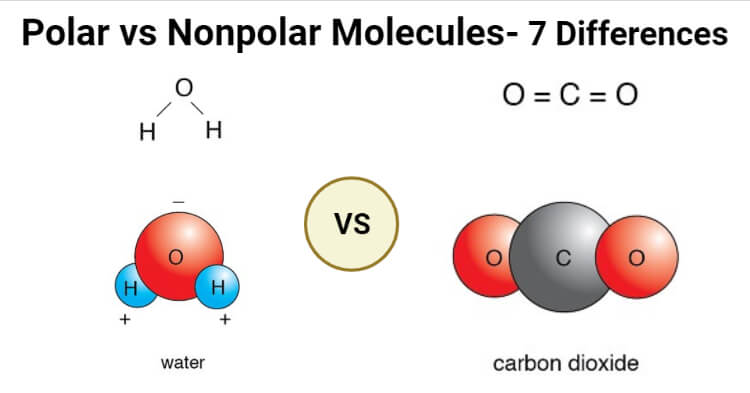
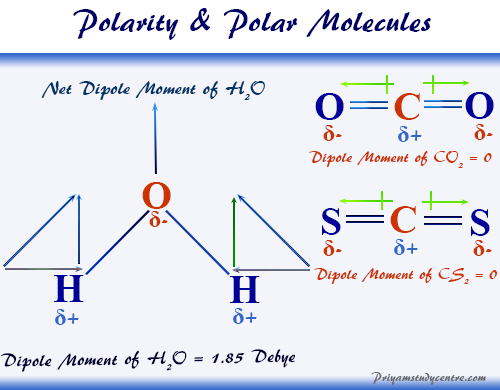
Carbon dioxide is a great example of how the geometry of a molecule plays a crucial role in determining whether its polar or nonpolar. Calculating the electronegativity. This makes a region of positive charge on the hydrogen atoms and the negative charge on the other end of the molecule which is the oxygen atom.
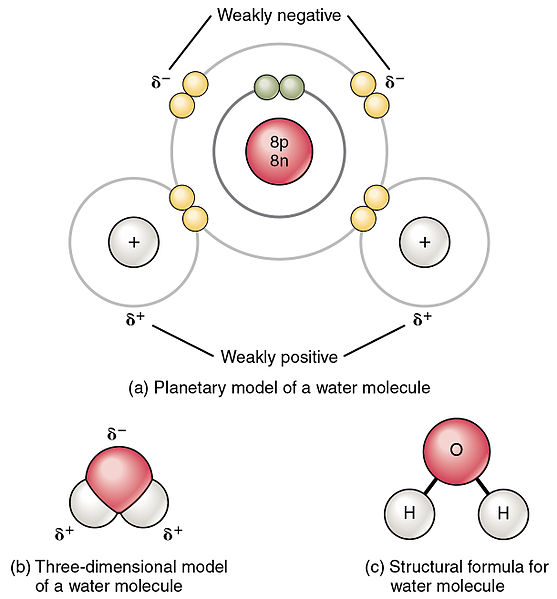
The polarity of any given molecule depends u. The electrons are unequally shared with the oxygen atom spending more time with electrons than the hydrogen atoms. Subsequently one may also ask is a water molecule polar or nonpolarWater is a polar molecule.
With its flat triangular geometry and D3h symmetry sulfur trioxide SO3 is a nonpolar molecule. Since electrons spend more time with the oxygen atom it carries a partial negative charge. So Is H2O polar on nonpolar.
The dipole moments within symmetrically formed molecules are essentially canceled out of each other due to their molecular geometry. It has no net dipole moment and any two consequent vectors S-O have moments that are identical in magnitude but opposite in direction to the third S-O moment. However to determine if H2O is.
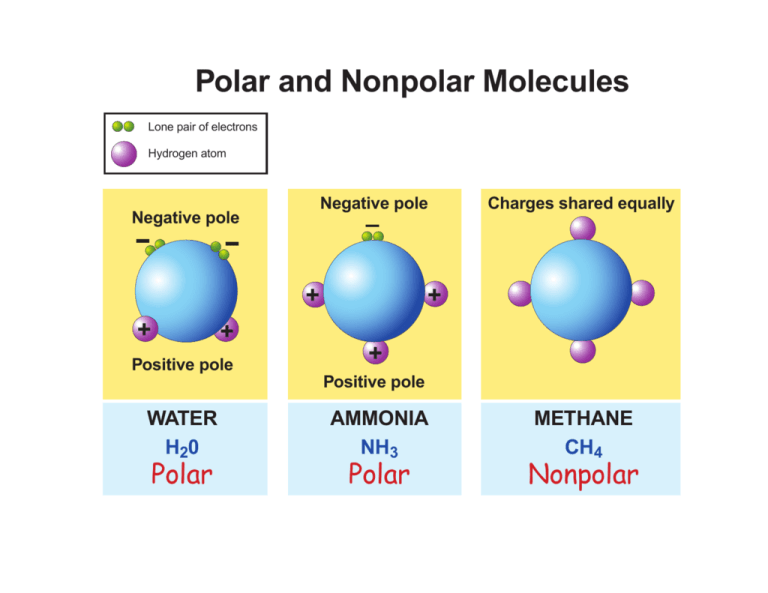
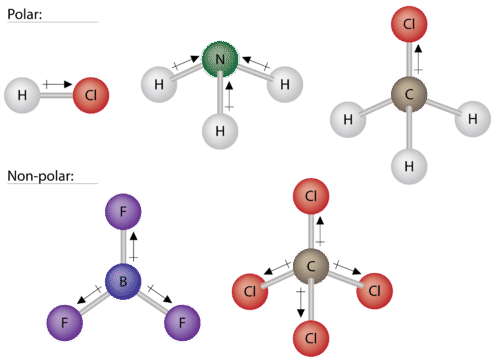
An H2 molecule is made up of two hydrogen atoms and they share the same electronegativity or tendency. 2 Is HCL polar or. Molecules with a twisted or asymmetrical form such as H20 and CH2Cl2 are more likely to be polar in nature.
Is h20 polar or non polar. Learn to determine if H2 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look and. This is because of the bent shape of the water molecule due to which there is an unequal charge distribution over the atoms of hydrogen and oxygen involved in the molecule of water.
Also know why is h20 polar covalent. 3-2 you can see that the two hydrogen atoms are not evenly distributed around the oxygen atom. The chemical formula of water is H20 which means that it contains two hydrogen atoms and one oxygen atom.
There actually are simple HCL is a polar molecule as chlorine has a higher electronegativity than the hydrogen. A water molecule abbreviated as H2O is an example of a polar covalent bond. Hey Guys In this video today we are going to look at the polarity of Ammonia having a chemical formula of NH3.
If you look at the Lewis structure for H2O we can see that it is not a symmetrical molecule. Learn to determine if H2 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look and. Thus it attracts electrons to spend more.
Why is SO3 Non-Polar molecule. 1 is a water molecule polar or nonpolar. Their electronegativity difference 038 is less than 05.
Due to Sulfur being more electronegative than Hydrogen it is partially negative. Each diagram shows the unsymmetrical shape of the water molecule. H20 water is a polar.
Is H2O Polar or Nonpolar bond and molecule and why Also how water is a Polar Covalent Molecule in Urdu Hindi for Class 9 10 icse Class 11-12 cbse Chemis. Because its oxygen is strongly electronegative and as such pulls the electron pair towards itself away from the two hydrogen atoms thus acquiring a slightly negative charge. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom.
Is a water molecule polar or nonpolar. Predicting The Polarity Of NH3 Molecule. When you look at a diagram of water see Fig.
What makes h20 a polar molecule. The EN difference between hydrogen and sulfur is 04 so hydrogen and sulfur form non-polar bonds. Is h20 polar or nonpolar.
H20 water is a polar. Sulfur trioxide SO3 is classified as a nonpolar molecule because of this. Weve gathered our favorite ideas for H20 Polar Or Nonpolar Explore our list of popular images of H20 Polar Or Nonpolar and Download Photos Collection with high resolution.
If the electronegativities are identical the bond will be completely non-polar. Its also important to understand the polarity of NH3 NH4 and polar vs nonpolar. Learn to determine if CH3COOH Acetic acid is polar or non-polar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Str.
However to determine if H2O is polar we need to look at the mo. The dipole moment is a vector value with both definite magnitude and direction. Therefore the water molecule.
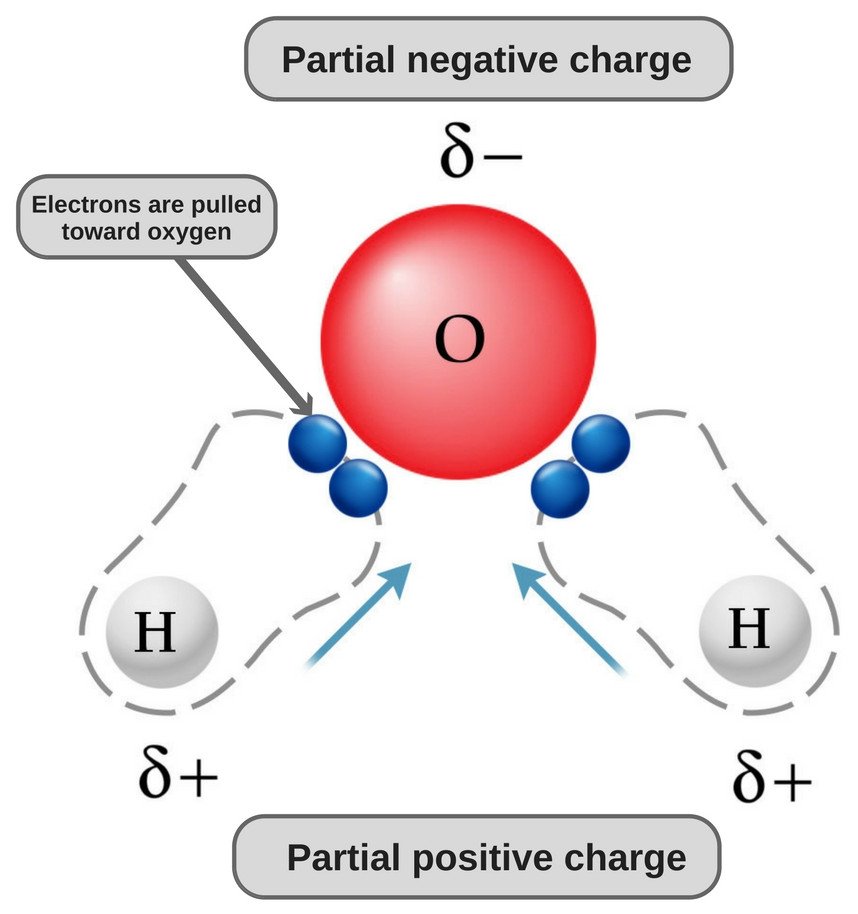
What is polar nature of water. Water H2O is a polar molecule because the electrons of the hydrogen atoms get pulled towards the electrons of the oxygen atom.

Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples Covalent Bonding Chemical Bond Study Chemistry

Is H2o Polar Or Nonpolar In Urdu Hindi Covalent Bond Chemicalbonding Youtube Youtube
Why Are The Bonds In H2o Polar Covalent Bonds Quora
Polar And Non Polar Molecules Biochemistry
Is Carbon Dioxide Co2 Polar Or Nonpolar Science Abc

Is H2o Polar Or Nonpolar Youtube

Polar Vs Nonpolar It S All About Sharing On An Atomic Level

Types Of Covalent Bonds Polar And Nonpolar Manoa Hawaii Edu Exploringourfluidearth

Is H2o Polar Or Nonpolar Water Youtube
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples
10 8 Electronegativity And Polarity Why Oil And Water Don T Mix Chemistry Libretexts
Polarity Of Bonds Polar Molecules Definition And Examples
Why Isn T Carbon Dioxide A Polar Molecule When Water Is A Polar Molecule Quora

Polar And Nonpolar Covalent Bonds Definitions Molecules And Examples
H2o Polar Or Nonpolar Check Covalent Bond And Polarity Geometry Of Molecules

Chemistry Time Polar Molecules

Is H2o Water Polar Or Nonpolar Techiescientist

Chemistry Ii Water And Organic Molecules