How To Tell The Shape Of A Lewis Structure
You do this by remembering VSEPR. The reactivity of a molecule and how it might interact with other molecules.

Im Dunkeln Leuchten Lewis Dot Diagramme Senior Chemie Chemie Chemie D Chemie Dot Organic Chemistry Study Teaching Chemistry Chemistry Lessons
For PCl 3 the electron dot diagram is as follows.

How to tell the shape of a lewis structure. Then with the Lewis structure we apply the. Because of the formula of the CCl4 molecule you can tell the carbon atom has four valence electrons. H 2 O 2 bonds.
For this molecule PF6 - the central atom is Phosphorus P. To a first approximation it doesnt matter whether the electron. If you are planning to draw a carbon tetrachloride CCI4 Lewis structure you will have to count all the valence electrons involved in each part of the overall molecule.
They do not provide information about the shape of the molecule. The steps that must be followed while drawing a Lewis structure are listed below. The P atom has four electron groups with three of them bonded to surrounding atoms so the molecular shape.
The Lewis structure helps us identify the type of bonding that may be present in a molecule based on the number of valence electrons available and the octet ruleThe octet. But whats usually missing is an understanding of what Lewis structures can tell us about molecules. A Lewis dot structure is a two-dimensional sketch of a molecule that uses dots to represent valence electrons.
Lewis structure is taught in conjunction with VSEPR Valence shell electron pair repulsion theory. The correct Lewis structure around an atom allows us to note the number of shared and lone electron pairs around a particular atom. Use this info to determine the 3D geometry of the molecule.
However once the Lewis structure is worked out you can easily work out the shape. H 3 O 3 bonds. Predicting Molecular Shape.
To predict the shape of the molecules first draw out the Lewis structure of the molecule. To determine the shapes of molecules we must become acquainted with the Lewis electron dot structure. Knowledge of Lewis structures can help us predict.
If the molecule is an anion extra electrons number of electrons added the magnitude of negative charge are added to the Lewis dot structure. The lone electron pairs on the Cl atoms are omitted for clarity. The shape of a molecule.
Once the total number of available electrons has been determined electrons must be placed into the structure according to these steps. The Lewis structure helps us identify the bond pairs and the lone pairs. Although the Lewis theory does not determine the shapes of molecules it is the first step in predicting shapes of molecules.
Draw the Lewis structure. Calculate the number of sigma σ bonds. You would start by drawing the Lewis Dot Structure and then count the number of bonds and lone pairs on the central atom.
Note the number of electron regions around the central atom and of these which are bonding or lone pairs non-bonding pairs Step 2. Since there are 4 bonds and 0 lone pairs the hybridization will be. Use the valence concept to arrive at this structure.
The Lewis structures in the previous section provide information about how a molecule is put together and how many bonding electrons and lone pairs they have. On the Lewis diagram identify the central atom. This step is crucial and one can directly get the state of hybridization and shape by looking at the Lewis structure after practicing with few molecules.
Concentrate on the electron pairs and other atoms linked directly to the concerned atom. First the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom. The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons on each individual atom.
The first step is to draw the Lewis structure of the molecule. These pairs of electrons arrange themselves in the shapes of the Platonic solids in the molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons.
The Lewis Dot Structure is a visual which represents the outermost shell of electrons also known as valence electrons and possible covalent bonds within an atom or molecule. Non-valence electrons are not represented in Lewis structures.

Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles Molecular Geometry Molecular Bond

Trigonal Planar Molecular Geometry Molecular Geometry Molecular Shapes Chemistry Projects

This 19 Page Worksheet Set Has Loads Of Lewis Structure Practice Molecular Shape Identification Pract Teaching Chemistry Chemistry Lessons Chemistry Classroom

Lewis Structure Teaching Chemistry Chemistry Classroom Chemistry Education

Co2 Lewis Structure Molecular Geometry And Hybridization Molecular Geometry Molecular Lewis

Shapes Of Molecules Bond Angles As Chemistry Chemistry Lessons Chemistry Education Organic Chemistry Study

From Gen Chem To Org Chem Pt 7 Lewis Structures Master Organic Chemistry Organic Chemistry Molecular Geometry Organic Chemistry Books

How To Find Valence Electrons And Total Electrons Youtube Chemistry Lessons Teaching Chemistry Chemistry Help
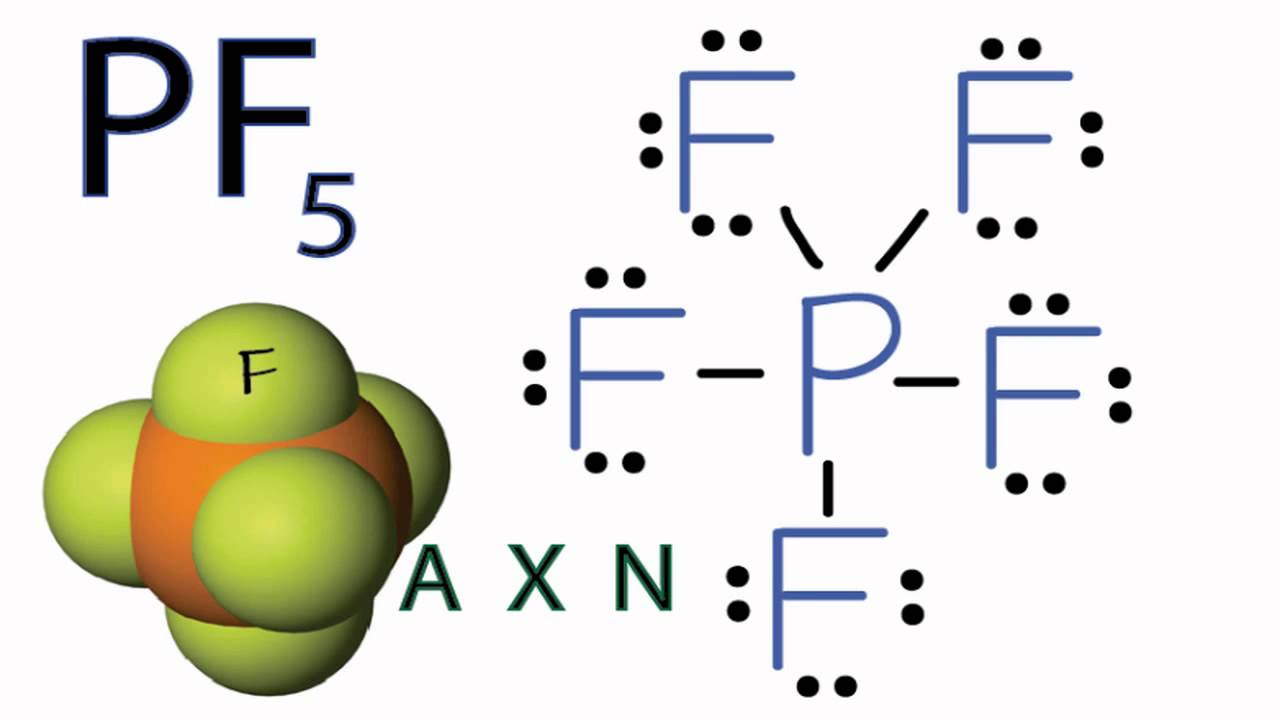
Pf5 Molecular Geometry Shape And Bond Angles Molecular Geometry Geometry Shape Molecular

Construction Of The Lewis Electron Dot Structure Of Cn2h2 Electrons Lewis Chemistry

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube Molecular Geometry Molecular Chemistry

Electron And Molecular Geometries Molecular Geometry Teaching Chemistry Chemistry

Steric Number And Bond Angles Molecular Geometry Teaching Chemistry Human Cell Structure

Lewis Dot Structures Worksheet Cheat Sheet Math Addition Worksheets Complex Sentences Worksheets Word Problem Worksheets

Lewis Dot Structure Worksheet Vsepr Origami Worksheet Post Lab Answers Kids Worksheets Printables Geometry Worksheets Math Addition Worksheets

Vsepr Theory Vsepr Theory Organic Chemistry Chemistry

Molecular Geometry Chart Molecular Geometry Molecular Geometry

