Is N2h4 Polar Or Nonpolar
Answer N2H4 Hydrazine is Polar What is polar and non-polar. If you look at the Lewis structure for C2H4 it appears to be a symmetrical molecule.

Hydrazine N2h4 Hydrazine Polar Molecule
Water H2O Nitrogen dioxide.
Is n2h4 polar or nonpolar. As a result the N-H bond is polar in the NH4 molecule. N2H4 is a very polar molecule. Question Is N2H4 polar or nonpolar.
Nonpolar molecules also form when atoms sharing a Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. N2H4 is a polar molecule with London dispersion forces dipole-dipole forces and hydrogen bonding between molecules whereas C2H6 is nonpolar and only has London dispersion forces between molecules. This problem has been solved.
Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. The difference between their electronegativity generates the polarity across the N-H bond. As a result the dipole of the molecule of Ethylene.
Total 2 lone pairs and 5 bonded pairs present in N2H4 lewis dot structure. Go look at the structure again. What determines how much products you can make in the chemical reaction.
N2H4 is polar in nature and dipole moment of 185 D. Polar or non polar. These are the most electronegative pieces in this atom.
Does n2h4 have a polar or nonpolar bond and ch4 is not a polar molecule. N2H4 is a polar molecule because the unshared electron pairs of the nitrogen atoms create an area on the molecule that is more negative than the space around the hydrogen atoms. Polar molecules show little ionic characteristics as they can conduct heat and electricity can be soluble in water and have a strong electrostatic force of attraction.
List molecules polar and non polar. This means that there is no way for their dipole moments to cancel. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
However to determine if C2H4 is polar we consider the molecular geomet. If there is only one line of symmetry the molecule is polar. C6H6 benzene is Nonpolar.
The net dipole moment shouldnt be zero in case of polar molecule because of the induction of partial positive and negative charges on either end of the molecule. The N-N bond is non-polar but the N-H bonds are polar hydrogen has a lower electronegativity than nitrogen has. Also only one line of symmetry can be drawn through the N2H4 dash model.
Learn to determine if PCl5 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look an. Ethylene C2H4 is nonpolar in nature because of the symmetrical linear geometrical shape. The formal charge on nitrogen in N2H4 is zero.
Each nitrogen in N2H4 has four pairs of electrons around it a lone pair a single bond to the other nitrogen and a single bond to each of the hydrogens. Polar Or Non Polar. Since N2O is linear and N2O is bent in shape so no need to check Electronegativity difference.
It is very polar. Ill tell you the polar or nonpolar list below. Answer N2H4 Hydrazine is Polar What is polar and non-polar.
The dipole value of NH4 ions is also non zero. Best Answer 100 1 rating Previous question Next question. Is n2h4 covalent or ionic.
Hydrazine - Wikipedia You can see here that 3 of the hydrogen atoms are below their respective nitrogen atoms and only one is above. What are anticlines and synclines. Electrons on the outer atoms are omitted for clarity.
In chemistry polarity refers to the distribution of electric charge around atoms chemical groups or molecules. VSEPR predicts a tetrahedral geometry for the electron pairs. Polar molecules must contain polar bonds due to.
A non polar molecule is a molecule that has an even distribution of electrons. Examples of Polar molecules. Another reason is that the hydrogen-carbon bonds are nonpolar because of nearly the same electronegativity.
If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. The electronegativity of Nitrogen is 304 and that of hydrogen is 22. In diatomic Iodine both atoms are the same so you would expect the molecule to be strictly non-polar.
Answer n2f2 is Nonpolar What is polar and non-polar. The difference in electronegativity between CarbonC 255 and HydrogenH 22 is 035 and therefore non-polar so yes the compound C2H2 is non-polar covalentmolecular.

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Why Doesn T N2h4 Form A Dative Bond Quora
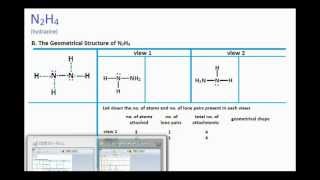
N2h4 Lewis Structure And Molecular Geometry Youtube

Monday January 6 Nd 3rd Period Ppt Download
Why Is Hydrazine N2h4 Polar It Seems To Me That The Sum Of The Left Side S Dipole Moments And The Right Side S Dipole Moments Are In Opposite Directions And Would Cancel Out
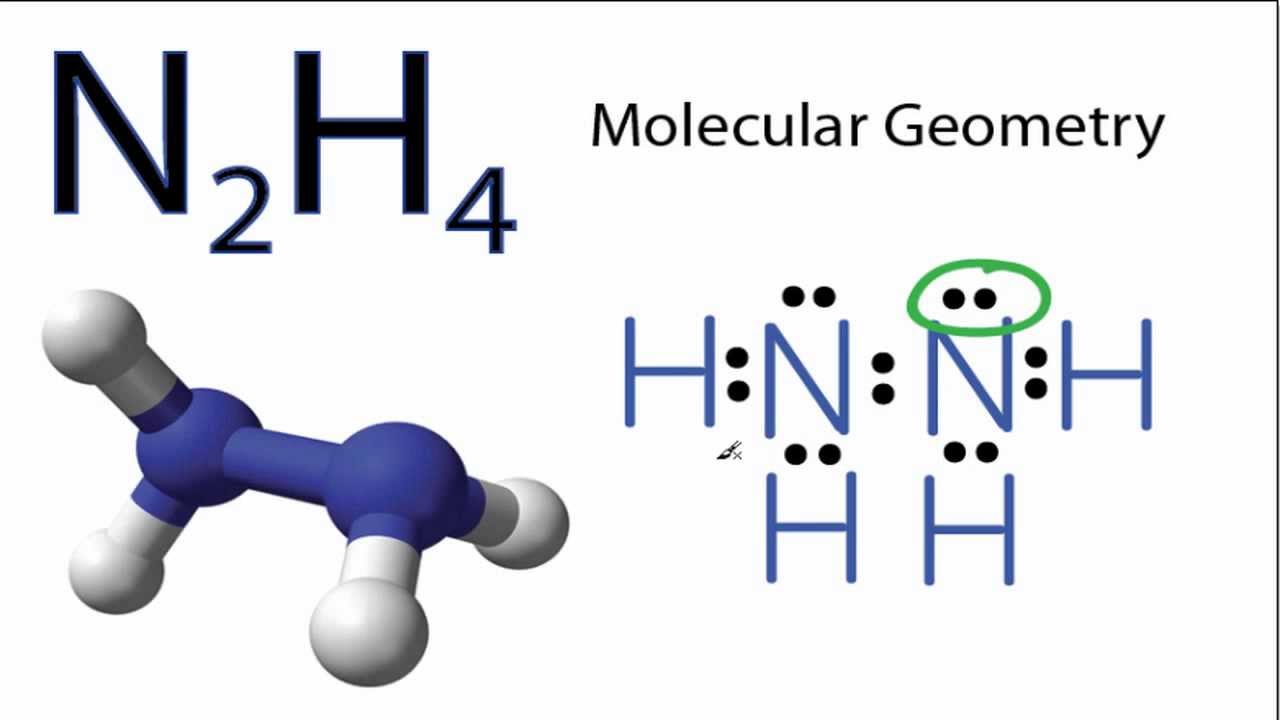
N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube

Complete The Following Chart For N2h4 Of Valance E Lewis Clutch Prep
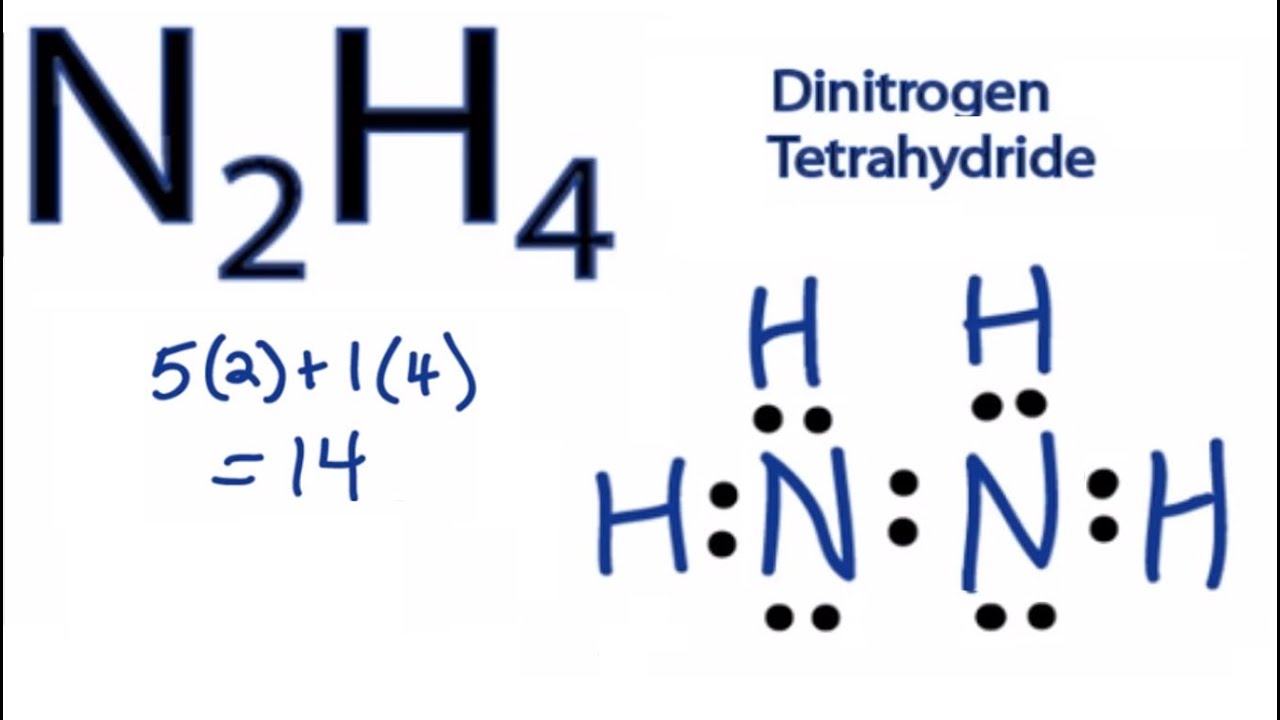
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube

Is C2h4 Polar Or Nonpolar Youtube

Identify The Kinds Of Intermolecular Forces That Might Arise Clutch Prep
Does N2h4 Have A Polar Or Nonpolar Bond And Molecule Quora

Draw The Lewis Structure For N2h4 Predict The Electron Geometry And Molecular Geometry And State Whether The Molecule Is Polar Or Nonpolar Study Com

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com
Hydrazine Intermolecular Forces Of Attraction

Ccl4 Lewis Structure Molecular Geometry Polar Or Non Polar Bond Angle




