Lewis Structure Of C2h2cl2
Reason for this is dipole moment. Indicate which of the compound s are polar.

There Are 3 Lewis Structures For C2h2cl2 D Clutch Prep
Total number of Valence electrons 4 21 27.

Lewis structure of c2h2cl2. The hybridization of the atoms in this idealized Lewis structure is given in the table below. A bonding orbital for C1-C2 with 19965 electrons __has 5000 C 1 character in a sp139 hybrid. The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2.
Learn this topic by watching Molecular Polarity Concept Videos. Carbons the least electronegative and well put that in the center and we know Hydrogens always go on the outside. Carbon has four valence electrons Hydrogen has one valence electrons and like all halogens Chlorine has seven valence electrons.
The valency of carbon is four so it likes to have exactly four bonds so if the each carbon atom is bonded to two non - carbon atoms the. Hybridization in the Best Lewis Structure. According to a classification scheme2 this estimated Koc value suggests that dichloroacetaldehyde is expected to have very high mobility in soil.
Using a structure estimation method based on molecular connectivity indices1 the Koc for dichloroacetaldehyde can be estimated to be 43SRC. Draw the Lewis Structure of the molecule C2H2Cl2. If you look at the Hydrogen atoms it only needs one valence electron to attain a stable structure.
In case of cis-12-dichloroethene and 11-dichloroethene the vectors are reinforcing each other perpendicularly to double bond hence giving a net dipole moment and making these two isomers polar in nature. Reason for this is dipole moment. In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds.
See the Big List of Lewis Structures. Trans-12-Dichloroethylene ClCHCHCl or C2H2Cl2 CID 638186 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. This is the C2H2Cl2 Lewis structure.
By signing up youll get thousands of step-by-step solutions to your homework. Hydrogen has 1 valence electron but we have two Hydrogens. For C2H2 Lewis structure we will first place both the Carbon atoms in the centre as it is less electronegative than the Hydrogen atoms.
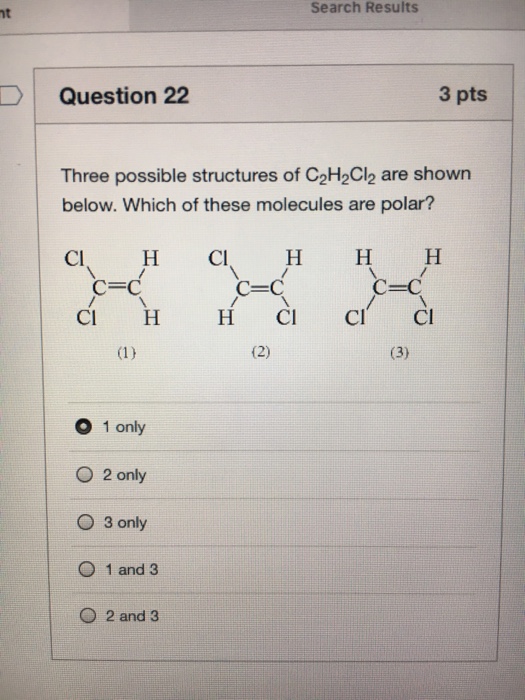
When we talk about CH2Cl2 Carbon is less electronegative than Chlorine atoms. A Draw Lewis structures of the three isomers all of which have a carboncarbon double. There are three possible structures isomers with the formula C2H2Cl2.
Explore more on itHereof why is C2H2Cl2 polar. Below are the three possible isomers of chemical formula C₂H₂Cl₂. Here both the Carbon atoms take the central position and the Hydrogen atoms are arranged around it.
Actually no there is only one acceptable Lewis structure for CHmath_2mathClmath_2math Moving the chlorines around does not produce a new compound with a. In case of cis -12-dichloroethene and 11-dichloroethene the vectors are reinforcing each other perpendicularly to double bond hence giving a net dipole moment and making these two isomers. Draw and explain the Lewis structure for eqC_2H_2Cl_2 eq.
The given molecule is a haloalkane molecule formed by the substitution of two hydrogen atoms of an ethene molecule. To understand the Lewis structure lets first calculate the total number of valence electrons for Dichloromethane. For the molecule we expect the carbons to be the central atoms in this molecule since carbon tends to be the central atom in its compounds.
Best Lewis Structure The Lewis structure that is closest to your structure is determined. There are three possible compounds isomers with the formula C2H2Cl2. Carbon has 4 valence electrons two Carbons.
Draw three Lewis structures for compounds with the formula C 2 H 2 F 2. So it is non-polar in natureOne may also ask is ch2ccl2 polar or nonpolar. Among these three isomers the trans -12-dichloroethene is non-polar while remaining two are polar.
Explain why one of these structures is non-polar and the other 2 are polar. Draw the Lewis structure with no dipole Two of the three structures can be interconverted by a process called cis-trans isomerization in which rotation around the central carbon-carbon bond takes place when the. There are 3 Lewis structures for C 2 H 2 Cl 2 draw all three and indicate whether each is non-polar or polar.
Plus Chlorine which is 7 and we have two Chlorines for a total of 24 valence electrons. You can smell very small amounts of 1 2-dichloroethene in air about 17 parts of 1 2-dichloroethene per million parts of air 17 ppmThere are two forms of 1 2-dichloroethene.
1 1 Dichloroethene C2h2cl2 Chemspider
There Are 3 Acceptable Lewis Structures For Ch2cl2 What Are The 3 Lewis Structures Quora
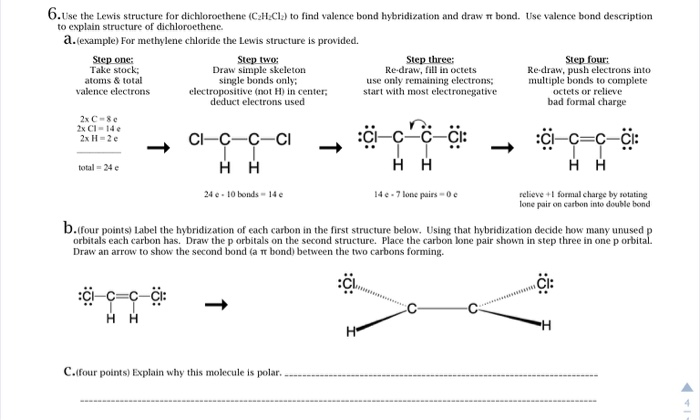
6 Use The Lewis Structure For Dichloroethene Cal Cl Chegg Com
Https People Chem Umass Edu Cmartin Courses Genchem F08 Exams Exam2 Eveningexam2b Anskey Pdf

State True Or False Two Isomers Of C2h2cl2 Are Polar
Search Results Nt Question 22 3 Pts Three Possible Chegg Com

Cis 1 2 Dichloroethene Structure C2h2cl2 Over 100 Million Chemical Compounds Mol Instincts

Why One Of The Three Structures For C2h2cl2 Is Nonpolar Brainly Com

C2h2br2 Lewis Structure How To Draw The Lewis Structure For C2h2br2 Youtube

C2h2cl2 Lewis Structure Homeworkavid
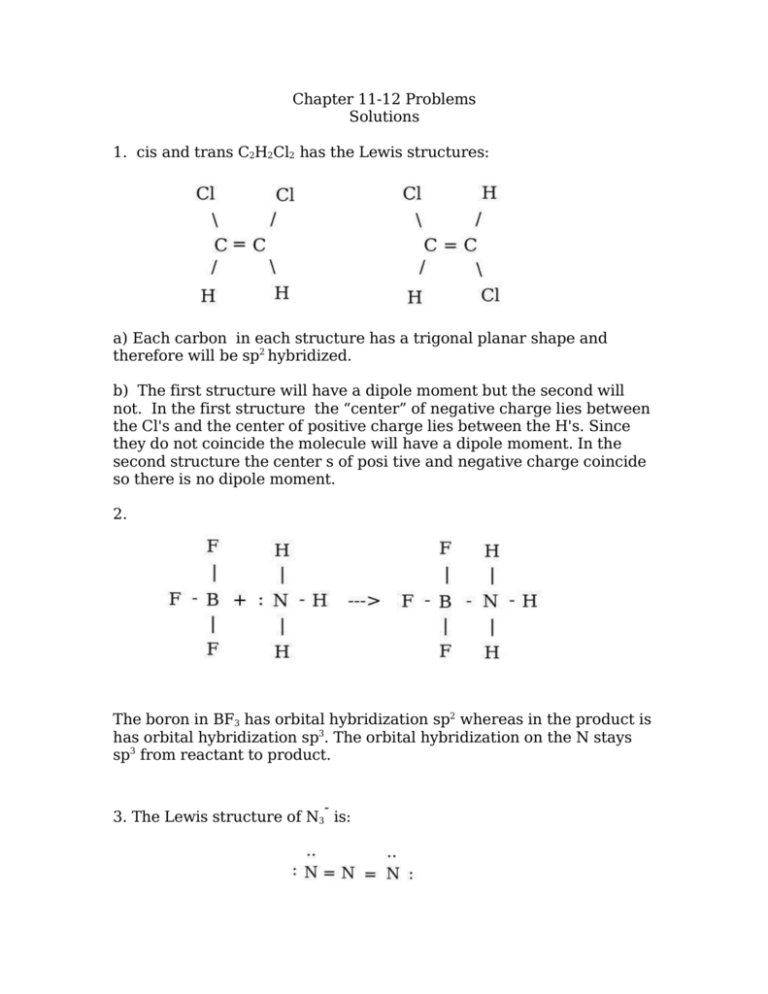
Chapter 11 12 Problems Solutions 1 Cis And Trans C2h2cl2 Has The
Laboratory 11 Molecular Compounds And Lewis Structures Flip Ebook Pages 1 10 Anyflip Anyflip

Dichloroethylene C2h2cl2 Has Three Forms Clutch Prep

Draw A Structure For The Trans Isomer Of C Clutch Prep

How To Determine The Lewis Dot Structure For C2h2cl2 Quora

C2h2cl2 Lewis Structure How To Draw The Electron Dot Structure For C2h2cl2
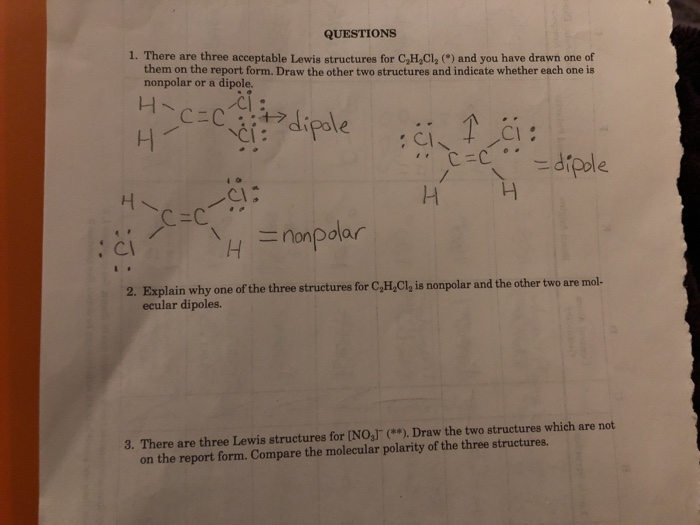
Explain Why One Of The Three Structures For C2h2cl2 Chegg Com

There Are 3 Lewis Structures For C2h2cl2 D Clutch Prep

There Are Three Different Dichloroethylenes Molecular Formu Clutch Prep